Core Benefit
- New BBB transportation technology of any CNS drug
- Very simple strategy; loosening tight junction of vascular endothelial cell and opening a way between the cells to deliver drug into brain.
Background and Technology
Drug transportation technology at Blood Brain Barrier (BBB) is a key to effectively use a CNS drug. Some methods are developing; glucose/amino acid modified drug transfers via transporter of vascular endothelial cell, conjugate consisted of a drag and an antibody against transferrin/insulin receptor internalizes into the cell. However, drug development requests minimization of invasiveness and enhancement of effectivity of transportation and specificity.
Here, I propose a new technology to transport drug via tight junction of BBB. This technology loosens the junction consisted of membrane proteins of vascular endothelial cells and makes a gap to transport drug molecular into brain. In contrast to the existing transportation technologies that need two steps (internalization of drug from blood to endothelial cell and release of the drug from the cell into brain), our method enables direct transportation of drug at the BBB and does not require any modification of drug.
Data and Publication
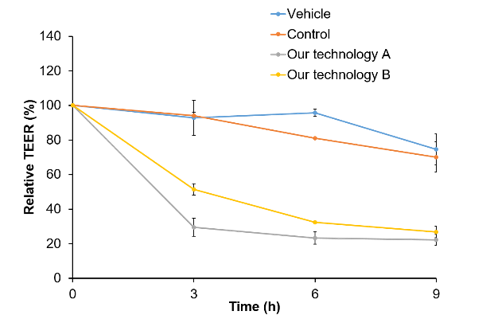
- In vitro transendothelial electrical resistance (TEER) measurement significantly showed our technology controls barrier of BBB.
- Similar measurement reveals the loosening of BBB is not permanent but temporal. As time passes more than half a day, function of the junction returns to its original state.
- In vivo experiment using cynomolgus monkey showed that our technology delivers tracer molecule from blood into cerebrospinal fluid.
Patent
PCT: WO2018105560A1
JP: 6900051B2
US: 10882906B2
EP: 3556771A4
Researcher
Yoshiaki OKADA Associate Professor (The University of Osaka, Department of Molecular Medicine)
Expectations
We are looking for a company to develop this technology using CNS drug delivery technology.
Product No: DA-01670