Advantages
- Reduce embryo wastage and improve the efficiency of assisted reproductive technologies (ART) treatment: Objectively quantify the risk of failed implantation for each cycle to help choose the most appropriate menstrual cycle.
- Low‑invasive, objective, quantitative evaluation: This probe-based measurement, which can be performed using a procedure equivalent to embryo transfer and can be used as a mock embryo transfer prior to the actual procedure, provides objective numerical data independent of the physician’s subjective judgment.
Current Stage & Data
Current development stage: A originally designed probe for intrauterine cavity measurement, combined with an existing body composition analyzer (Fig. 1), has obtained PMDA approval under Japan’s “rebalancing” notification framework.
- Uterine receptivity assessment: In the clinical study (a prospective observational study in the frozen‑thawed single embryo transfer cycles), the measurement value of in-vivo intrauterine bioimpedance predicted non‑pregnancy in that cycle (AUC = 0.88) (Fig. 2).
Next Step: Plan a clinical trial to verify improvements in ART efficiency using this system.
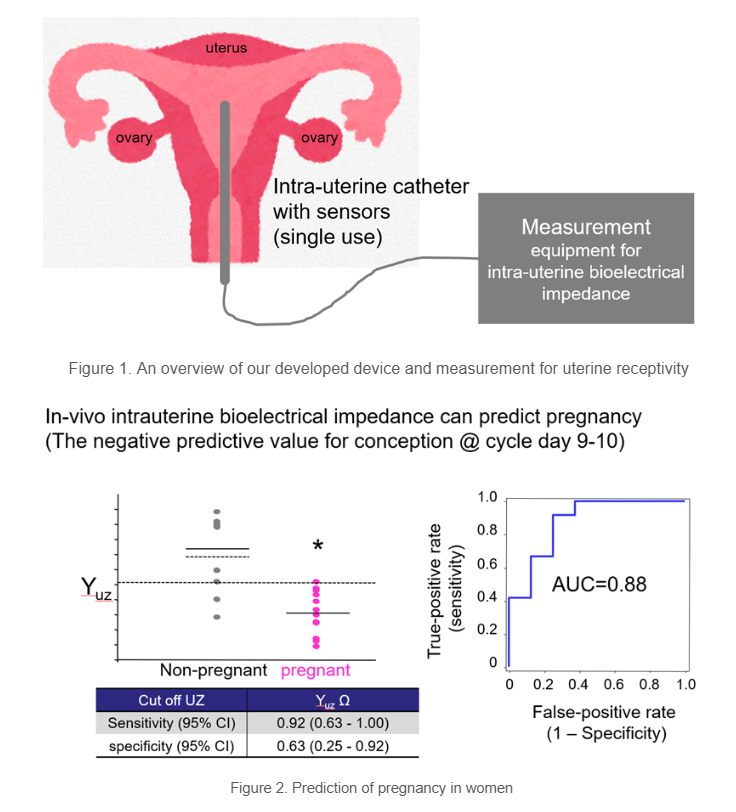 |
Technology Overview & Background
ART treatment is common in worldwide and in high demand. However, it is not efficient enough. Pregnancy only occurs when certain conditions are met on both the egg and uterine sides. However, there was no adequate method of prospectively evaluating uterine receptivity in each menstrual cycle. The problem with current ART treatments is that the number of embryos is limited, and they are not used efficiently without an adequate assessment of uterine receptivity. Focusing on changes in mucosal components such as glycans and glycolipids of the endometrial epithelium associated with implantation, the team developed a disposable, sensorized probe (catheter) that measures bioelectrical impedance on intrauterine tissue surfaces, capturing molecular‑level changes during receptivity priming as objective numeric features.
Partnering Model
The University of Osaka seeks companies interested in commercialization of this medical device. As a next step, a meeting with the researchers is proposed; please contact us to discuss further.
Publication(s)
- Nakamura H et al. (2018) Reprod Med Biol. 23;17(3):255-261.
DOI: https://doi.org/10.1002/rmb2.12098
- Nakamura H et al. (2018) Hum Reprod. 33(12):2241-2248.
DOI: https://doi.org/10.1093/humrep/dey313
- Nakamura H et al. (2018) Reprod fertil dev. 30(4) 619-623.
DOI: https://doi.org/10.1071/RD17209
Patent(s)
- PCT/JP2015/001708 (Registered in US and JP)
- PCT/JP2024/019026 (Pending in US and JP)
Principal Investigator & Academic Institution
Assistant Prof. Hitomi NAKAMURA (Graduate School of Medicine,The University of Osaka)
Project ID:TT-00614


