Advantages
- Higher accuracy than visual and palpable examination
- Sample collection is minimally invasive, placing less of a burden on patients than mammography
Current Stage and Key Data
Partnering Model
Seeking partners to license and develop the breast cancer biomarkers
- Examples of potential partners: diagnostic drugs, diagnostic equipment, clinical testing services, LDT service companies
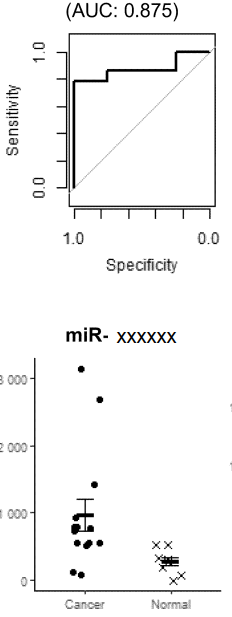
Background
Breast cancer is a malignant tumor that develops in the breast tissue. It is the most commonly diagnosed cancer in women, although it can also affect a small number of men. Early detection significantly improves patient outcomes, and breast cancer screening is therefore common in Japan. Breast cancer screening is typically performed by visual and palpable examination and mammography. However, issues with the accuracy of visual and palpable examination have been pointed out, and issues with mammography include pain during the examination and radiation exposure. For these reasons, the development of new biomarkers is needed.
The nipple discharge that is the subject of testing in this invention is nipple secretion fluid (non-milk) from times other than the lactation period (the period from birth to weaning), and is a liquid that is naturally secreted from the nipple or obtained by applying pressure to the breast.
Patents and Publications
- Paper submission in preparation, patent application filed (unpublished).
Principal Investigator
Akira Yokoi (Department of Obstetrics and Gynecology, Nagoya University Hospital, Tokai National Higher Education and Research System)
Project ID:BK-05194