Advantages
- Novel specific biomarkers for refractory and poor prognosis GAS.
- Repositioning therapeutic drugs targeting the biomarker-related pathways.
Current Stage and Key Data
Biomarkers identified by using human samples, and therapeutic drug efficacy confirmed in animal experiments.
- Biomarkers were identified through analysis of tissue samples from patients with GAS, usual type endocervical adenocarcinoma (UAE), and lobular endocervical glandular hyperplasia (LEGH). Immunohistochemical staining of the biomarker confirmed its usefulness as a GAS-specific marker.
- Existing small molecule therapeutic drugs showed growth-inhibitory effects in cultures of GAS cell lines established from human GAS patients and in CDX model mice subcutaneously implanted with the same cell lines.
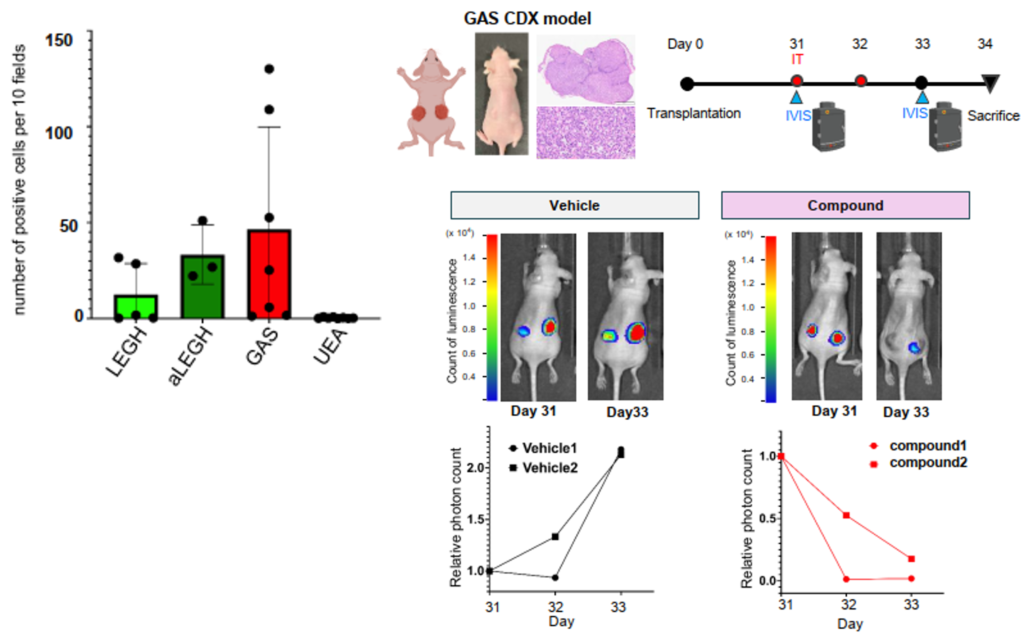 |
Partnering Model
- We are looking for partners to collaborate on the development of cancer diagnostics, companion diagnostics, and anti-cancer drugs.
- Potential partner examples: diagnostic, LDT service, pharmaceutical/biotech companies.
Background
GAS is a type of HPV-independent mucinous cervical carcinoma, accounting for approximately 20-25% of cervical adenocarcinomas (approximately 20% of all cervical cancers). Compared to UEA, GAS is more resistant to chemotherapy and radiotherapy and has a poorer prognosis, so early diagnosis and treatment are desirable. However, existing markers cannot distinguish from UEA or the benign LEGH tumor, so the development of a GAS-specific diagnostic marker is desired.
The first-choice treatment strategy for GAS is hysterectomy, which is highly invasive. When GAS is discovered at an advanced stage or in cases where surgery is not possible, chemotherapy and radiation therapy are performed, but GAS often exhibits resistance to these therapies, and the development of more effective treatments is desired. Clinical trials of molecular targeted therapies have been attempted, primarily targeting cases of breast cancer, gastric cancer, and RAS-mutated gynecological cancers, but also including GAS patients, but sufficient therapeutic effects against GAS have not been achieved.
Patents and Publications
- Paper submission in preparation, patent application filed (unpublished).
Principal Investigator
Akira Yokoi (Department of Obstetrics and Gynecology, Nagoya University Hospital, Tokai National Higher Education and Research System)
Project ID:BK-05195


