Advantages
- ALS diagnosis and precision medicine
Current Stage and Key Data
Target validation and seed compound identification
- Identification of miRNAs significantly decreased in EVs from ALS patients compared to serum from healthy individuals
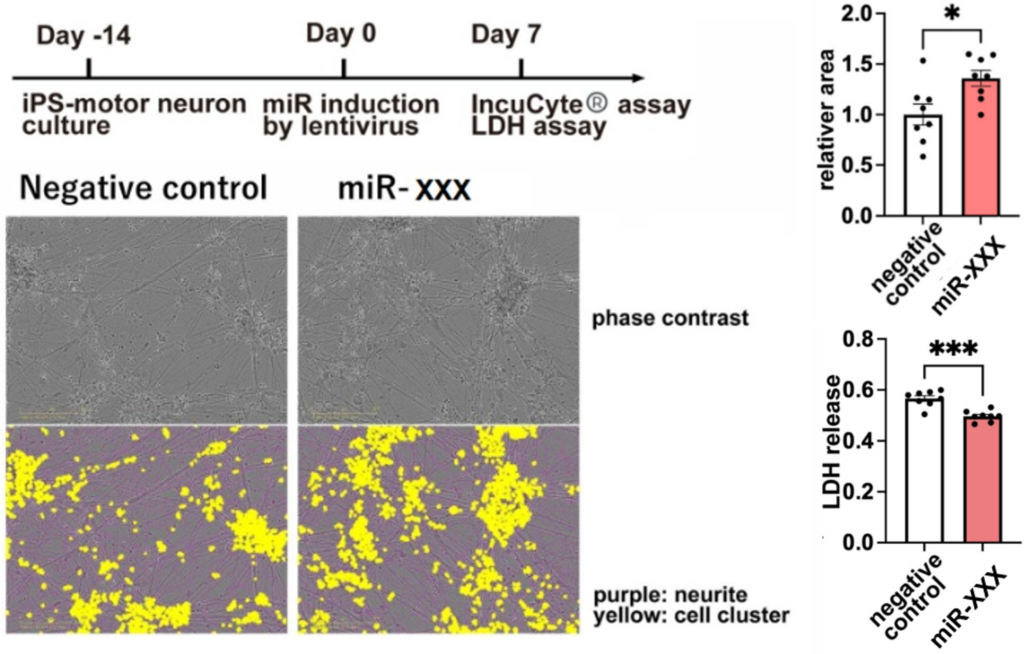
- Transfection of the miRNA into ALS model cells (SH-SY5Y/TDP-43A315T) reduced toxicity and restored viability (WST, LDH assay).
- Pathway analysis revealed that the miRNA suppressed p53 activity and inhibited apoptosis.
- When the miRNA was introduced into motor neuron cells derived from iPS cells of ALS patients using a lentivirus, the reduction in cell cluster area and cytotoxicity were suppressed.
 |
Partnering Model
Seeking partner companies to license and develop this technology.
- Potential partners: Pharmaceutical/biotech companies focusing on ALS treatment drug discovery , miRNA drug discovery, gene therapy, exosome therapy, and/or diagnostic marker development
Background
ALS is a severe neurodegenerative disease characterized by the progressive degeneration and damage of motor neurons in the nervous system, ultimately leading to paralysis and death in affected individuals. ALS is often sporadic, and there are no reliable diagnostic indicators. The pathogenesis of ALS is also poorly understood, and with the exception of treatments for ALS caused by SOD1 gene mutations, only symptomatic treatments are available, so the development of a fundamental treatment is needed.
Patents and Publications
- Patent pending
- Paper submission in preparation
Principal Investigator
Masahisa Katsuno (Graduate School of Medicine, Nagoya University, Tokai National Higher Education and Research System)
Project ID:BK-05377


