Advantages
- Patient-derived CNS evidence: Using single-nucleus RNA sequencing of motor cortex and spinal cord tissues from ALS patients, significant upregulation of mGluR5 was newly identified.
- Highly original analytical approach: Single-nucleus level analysis of patient-derived central nervous system samples remains technically demanding and globally rare. This study provides the first scientific validation of abnormal mGluR5 expression in ALS.
- Specific upregulation in spinal motor neurons: Marked mGluR5 elevation was specifically observed in spinal motor neurons—the major site of motor dysfunction in ALS—suggesting a strong mechanistic link.
- Neuroprotective effect of mGluR5 inhibition: In ALS model cells, suppression of mGluR5 expression improved neurite outgrowth defects.
Technology Overview & Background
ALS is a progressive neurodegenerative disease affecting upper and lower motor neurons, leading to muscle weakness and atrophy. With no fundamental cure currently available, novel molecular targets are needed.
Abnormal accumulation of TDP-43 protein—present in approximately 97% of ALS patients—is tightly associated with motor neuron degeneration. Both cell-autonomous mechanisms (within motor neurons) and non-cell-autonomous mechanisms (involving glial and vascular cells) are thought to drive ALS pathogenesis.
ALS is therefore a complex, multicellular disorder rather than one confined to a single cell type. Because the molecular interactions among these cell types remain poorly understood, analyses that address both motor neurons and their microenvironment—such as in the present study—are expected to be essential for uncovering disease mechanisms and identifying therapeutic targets.
Through deep profiling of ALS patient tissues exhibiting TDP-43 pathology, mGluR5 was found to be markedly upregulated. Neuronal models with reduced TDP-43 expression exhibited severe neurite shortening, which was rescued by mGluR5 inhibition, confirming a neuroprotective role. Because mGluR5 is mainly expressed in the central nervous system, its inhibition is also expected to pose minimal systemic side effects—offering favorable clinical potential.
Experimental Data
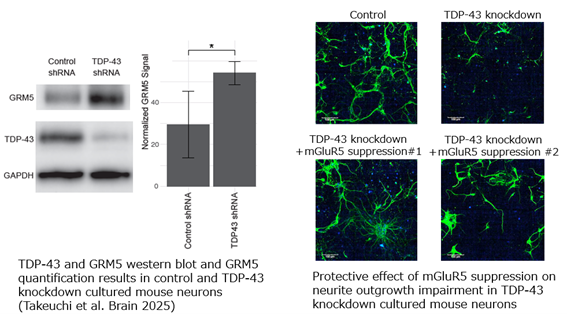
- TDP-43 knockdown (via shRNA) in cultured mouse neurons caused dramatic neurite shortening.
- In the same cells, shRNA-mediated suppression of mGluR5 restored neurite elongation, indicating neuroprotection.
 |
Patent(s)
Applied (Unpublished)
Principal Investigator & Academic Institution
Specially Appointed Prof. Seiichi NAGANO (United Graduate School of Child Development, The University of Osaka)
Ongoing and Planned Studies
- Evaluation of mGluR5 inhibition efficacy in in vivo ALS models
- Screening of existing and novel mGluR5 inhibitors for therapeutic potential
- Discovery and development of novel mGluR5-targeted compounds
Collaboration Opportunity
The University of Osaka is seeking partnerships with pharmaceutical companies interested in developing ALS therapeutics targeting mGluR5, through licensing or collaborative research.
As the next step, we would like to propose a meeting with the researcher. For more information or partnership inquiries, please contact us to explore collaboration opportunities.
Project ID:TT-05261


