Advantage and Core Benefit
- Significantly reduces the risk of non-specific binding and photoactivation of residual drugs in the blood by natural light, potentially converting the current one-week inpatient treatment into outpatient treatment.
- Concept technology with the potential to be universally applicable to a variety of antibodies and sensitizers.
Background and Technology
Photoimmunotherapy (such as Aluminox™) involves accumulating a photosensitizer-antibody conjugate in targeted cancer cells and then irradiating them with near-infrared light to generate reactive oxygen species, which specifically destroy the cancer cells.(*) Because it can theoretically be applied to a wide range of cancers depending on the antibody selected, it is expected to become a “fifth treatment” following surgery, chemotherapy, radiation therapy, and immunotherapy. However, the biggest bottleneck in widespread adoption is the risk of phototoxicity from residual drugs. Current protocols require approximately one week of strict darkroom hospitalization to avoid phototoxicity, which increases patient burden and medical costs.
This technology fixes a photosensitizer and quencher connected by a linker to the N-terminus of the antigen-binding site. The linker is exposed only upon binding to the antigen, allowing the quencher to be cleaved by hydrolysis. Drugs in the blood and drugs nonspecifically bound to the Fc region are not activated, fundamentally suppressing phototoxicity (fig.).
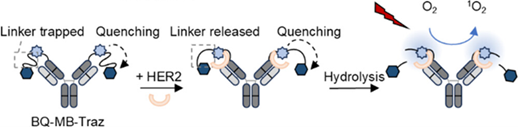 |
*Current photodynamic therapy damages cells with reactive oxygen, whereas Aluminox differs in that the photosensitizer becomes hydrophobic when its hydrophilic groups are released by reactive oxygen, causing it to aggregate and destroy the cell membrane, killing the cells.
Data
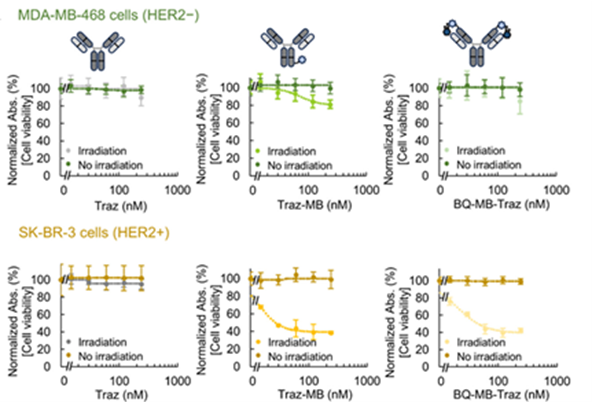
- A conjugate was created using methylene blue (MB) and black hole quencher 3 (BQ) as the photosensitizer and quencher, respectively, and trastuzumab (HER2 antibody: Traz) as the antibody (this technology: BQ-MB-Traz). As a control mimicking the current drug, an antibody (MB-Traz) was created in which the photosensitizer was immobilized on the Fc region.
- BQ-MB-Traz demonstrated the same cytotoxic effect as MB-Traz in HER2-positive cells (fig. bottom: center and right). Meanwhile, while MB-Traz exhibited non-specific toxicity against HER2-negative cells, BQ-MB-Traz maintained high selectivity (fig. top: center and right).
 |
Patent & Publication
Patent pending from Institute of Science Tokyo (JP2025-57686:Unpublished)
ACS Omega 2025, 10, 24, 25596–25604(https://doi.org/10.1021/acsomega.5c01083)
Researcher
Associate professor, Tetsuya Kitaguchi (Laboratory for Chemistry and Life Science, Institute of Science Tokyo)
Expectations
We are seeking biotech/pharmaceutical companies to jointly develop next-generation photoimmunotherapy drugs under a license for this technology.
Phase 1: In-vivo proof of concept (company)
Phase 2: Selection of target antibody and photosensitizer (company), conjugate design, production, and optimization (company-university collaboration)
Phase 3: Conjugate performance evaluation (company)
*Pre-prepared conjugates can be provided for initial evaluation (paid).
Project ID:KJ-05379


