Advantages
- More effective companion diagnostics and therapeutic agents for FLT3 inhibitor-resistant AML.
Current Stage and Key Data
We have identified FLT3 inhibitor resistance markers and candidate resistance-improving agents, and confirmed their effectiveness in a human leukemia cell model.
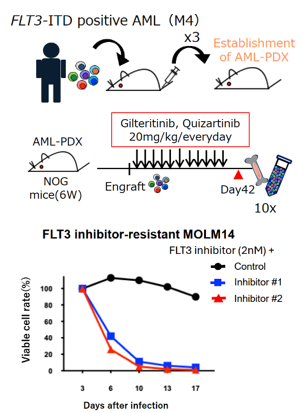
- PDX mouse models were created by transplanting bone marrow cells collected from FLT3 gene mutation-positive AML patients. We identified a group of molecules that were more highly expressed in leukemia cells remaining after long-term daily administration of FLT3 inhibitors (resistance-acquired) than before administration (top figure).
- High expression of the same group of molecules was also confirmed in the FLT3 mutant human leukemia cell line MOLM14, which had acquired resistance.
- Increased expression of one of the molecules in the same group was observed in patient cells that had clinically become resistant to FLT3 inhibitor treatment.
- In resistant MOLM14, the same group of molecules was inhibited with a candidate resistance-improving agent. Sensitivity to FLT3 inhibitors was restored (bottom figure).
 |
Partnering Model
We are seeking partnerships with companies focused on the development of AML diagnostics and treatments, and are open to collaborative research and commercial licensing.
- Examples of potential partners: Pharmaceutical/biotech companies and diagnostic companies focused on developing and providing treatments for blood cancer and AML, as well as FLT3 inhibitors.
Background
Constitutively active mutations in the FLT3 (Fms-Like Tyrosine Kinase 3) gene are a poor prognostic factor found in approximately 20% of acute myeloid leukemia (AML). For this reason, various FLT3 inhibitors are used to treat AML; however, resistance to FLT3 inhibitor therapy can develop relatively quickly. For example, in clinical trials of the FLT3 inhibitor gilteritinib in relapsed/refractory FLT3 mutation-positive AML, median survival was reported to be only 9.3 months with gilteritinib treatment, compared with 5.6 months with salvage chemotherapy, highlighting the short duration of response to FLT3 inhibitors.
Patents and Publications
- Paper submission in preparation, patent pending (unpublished).
Principal Investigator
Professor Hitoshi KIYOI (Graduate School of Medicine, Nagoya University, Tokai National Higher Education and Research System)
Project ID:BK-05208


