Advantages
- Rising clinical / research demand for identification technology for MOGAD and MS (Multiple Sclerosis)
- Simple process using an undisclosed exosome-derived protein, SLAMF6(CBA method not required)
- SLAMF6 strongly correlates with the IgG index, a specific marker of MOGAD
Background & Technology
Multiple sclerosis (MS), neuromyelitis optical spectrum disorder (NMOSD), and MOG antibody-associated disease (MOGAD) are all autoimmune diseases of the central nervous system. They are caused by the destruction of the myelin sheath that covers nerve fibers in the brain and spinal cord. These diseases can cause various symptoms such as limb paralysis, blindness, and sensory disturbances, and can lead to significant disability, even in young people. Since the treatment and prognosis for each disease are very different, and there is a high risk of relapse, it is important to make an accurate and rapid diagnosis in the early stages.
The presence of MOG antibodies is essential for diagnosing MOGAD, but current diagnostic criteria rely on a special method called the Live-cell based assay (CBA), which is not easy to perform. In addition, diagnostic sensitivity is often insufficient depending on the sample, as some cases are negative in serum but positive in cerebrospinal fluid (CSF).
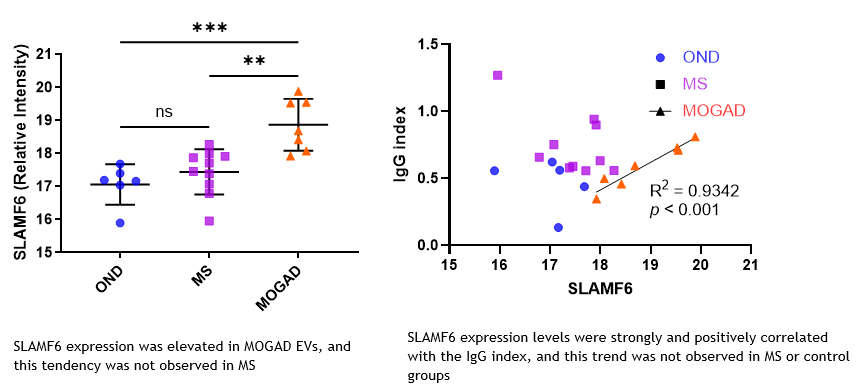
To solve these problems, a new diagnostic marker, SLAMF6, was discovered through proteome analysis of exosome-derived proteins in cerebrospinal fluid at Sapporo Medical University. This molecule is highly expressed in exosomes from the CSF of MOGAD patients, but this trend is not seen in MS patients. Furthermore, the expression level of SLAMF6 shows a strong positive correlation with intrathecal IgG production (IgG index), which is a characteristic of MOGAD. This suggests that SLAMF6 may be able to differentiate between MOGAD and MS with high sensitivity and specificity. It is also hoped that SLAMF6 can be measured with a simpler ELISA method, which is expected to contribute to the future development of diagnostic technology.
Therefore, this technology is particularly useful for diagnosing cases with low MOG antibody titers or those that are only positive in CSF, as well as for clinical research. There is a need for the establishment and research and development of this technology for practical use as a standard diagnostic method for MOGAD and as a simple evaluation item to supplement existing MOG antibody measurement methods.
Data
SLAMF6 Expression Results
Left: Results of expression analysis after isolating extracellular vesicles (EV) from the cerebrospinal fluid (CSF) of each patient and eluting them for proteomic analysis.
Right: Comparison of expression levels and IgG indices in CSF among each patient group.
 |
Patent
Pending (unpublished): JP2025-072434 (Filing Date: April 24, 2025)
Applicant
Sapporo Medical University
Researcher
Masanobu Tanemoto, Naotoshi Iwahara, Shin Hisahara; Department of Neurology, Sapporo Medical University
Ippei Ikegami, Shingo Ichimiya; Department of Human Immunology, Research Institute for Immunology, Sapporo Medical University
Jun Adachi; Laboratory of Proteomics for Drug Discovery, Center for Drug Design Research, National Institute of Biomedical Innovation, Health and Nutrition
Development Phase
Current status: Concept verification of high-sensitivity differentiation between MOGAD and MS using SLAMF6 has been completed.
Next step:
- Accumulation of diagnostic validation data of SLAMF6 in blood samples using ELISA.
- Validation of the ability of SLAMF6 expression to measure recurrence using clinical samples.
We are seeking partner companies interested in collaborative research for the two steps above and subsequent development. We would be happy to start with a detailed explanation and discussion of the technology.
Project ID:ON-05334


