Advantages
- Quantitative evaluation of the reproductive toxicity (toxicity to spermatogenesis) of compounds over time.
- Reducing the number of animals used to evaluate the reproductive toxicity of pharmaceuticals.
Current Stage and Key Data
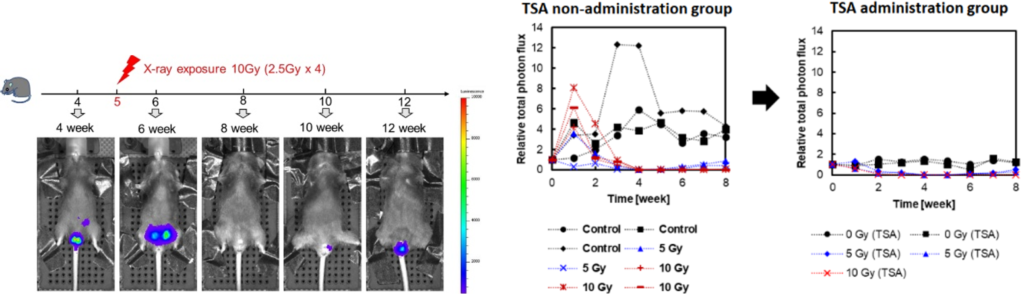
Male reproductive toxicity test model mice have been established (left image)
- An example of testing reproductive toxicity (spermatogenesis failure) caused by the histone deacetylase inhibitor trichostatin A (TSA) using this model (right image).
 |
Partnering Model
We hope to license this model mouse and testing method to companies that conduct reproductive toxicity tests on pharmaceutical compounds and other substances, and to companies that provide experimental animals. Joint research is also possible.
- Potential partners include pharmaceutical/biotech companies, non-clinical CRO companies, laboratory animal companies, and agricultural chemical, food, and cosmetic companies.
Background
Reproductive toxicity is a phenomenon that alters the reproductive ability of parents or their offspring (including morphological and functional abnormalities, as well as damage to the embryo and fetus) due to exogenous chemicals, physicochemical factors, or endogenous physiological factors. In conventional one-generation reproductive toxicity tests, parental male and female experimental animals are typically administered, for example, chemicals, and then mated to confirm fertility (reproductive toxicity) and observe the effects on the development of the offspring up to sexual maturity. If necessary, the gonads are sometimes removed after euthanasia and subjected to histopathological examination.
The present invention provides a method for quantitatively evaluating reproductive toxicity over time using a model mouse into which a reporter gene for a gene that is specifically expressed in spermatogenic cells has been introduced.
Patents
- Patent pending
Principal Investigator
Hisanori FUKUNAGA (Graduate School of Health Science, Hokkaido University)
Project ID:BK-05351


