Advantages
- Higher specificity for individual patient cancer antigens than conventional TCR-T/NK cell therapy.
- Easier to produce than TIL (Tumor Infiltrating Lymphocyte) therapy.
 |
Current Stage and Key Data
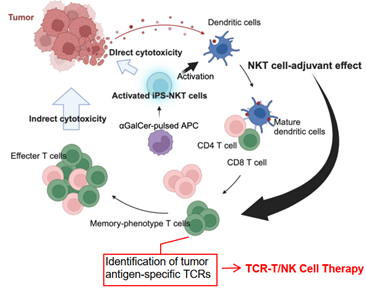
- It has been confirmed that personalized TCRs can be efficiently identified by stimulating the patient’s peripheral blood cells with optimized NKT cells to induce and collect anti-tumor T cells.
- The therapeutic effect of induced anti-tumor T cells has been confirmed.
Partnering Model
We are seeking partnerships with companies developing cancer immune cell therapies. We are open to joint research and commercial licensing.
- Potential partner examples: Pharmaceutical/biotech companies focusing on NTK cell therapy, TCR-T/NK cell therapy, and CAR-T/NK cell therapy.
Background
TCR-T/NK cell therapy, known as cancer immunotherapy, has the advantage over CAR-T cell therapy of being able to target intracellular molecules and being specific for tumor-specific peptides. However, while the TCRs were created by identifying peptides from the tumor’s overall genomic information, it was unclear whether they would match specific patients and tumors. Furthermore, TIL (Tumor Infiltrating Lymphocyte) therapy, known as a patient-specific T cell therapy, involves collecting lymphocytes that have infiltrated the patient’s tumor tissue, isolating, culturing, and expanding activated anti-tumor T cells, and then returning them to the body. However, the isolation, culturing, and expansion of activated anti-tumor T cells make for a complex production process.
Publications and Patents
- Paper submission in preparation and patent pending (unpublished).
Principal Investigator
Takahiro AOKI (Medical Immunology, Graduate School of Medicine, Chiba University)
Project ID:BK-05227


