 |
Advantages
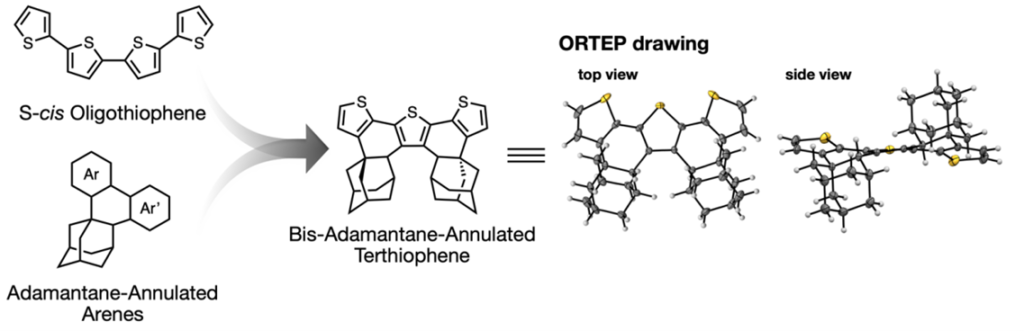
- A method for synthesizing oligothiophene compounds with s-cis conformation, which has been difficult to synthesize until now.
- Higher solubility and electronic conductivity compared to conventional oligothiophene compounds with s-trans conformation.
- Promising organic electronic material.
Current Stage and Key Data
- Established a method for synthesizing adamantane-annulated oligothiophene compounds with s-cis conformation in the laboratory
- Calculation of charge transfer integrals and analysis of absorption and fluorescence spectra
Partnering Model
We are currently seeking partner companies to evaluate, apply, and develop new compound materials based on this technology in the fields of organic semiconductors and electronics. Samples of this compound can be provided. Please contact us regarding sample size.
- Examples of potential partners: organic semiconductor material companies, organic electronics companies, chemical companies, etc.
Background
Oligothiophenes and polythiophenes (e.g., P3HT) have excellent electronic conductivity and mechanical strength and are widely used in the field of organic electronics. However, thiophene rings usually tend to adopt the s-trans conformation, which has low conductivity, and it has been difficult to stabilize the s-cis conformation, which has higher conductivity. To solve this problem, we developed a technology to fix the thiophene ring in the s-cis conformation by fusing an adamantane skeleton to the oligothiophene. This technology is expected to be applied to a variety of organic electronic materials.
Patents and Publications
Patent: PCT international patent pending, and Paper: Preparing for submission.
Principal Investigator
Professor Akiko Yagi (Graduate School of Science, Nagoya University, Tokai National Higher Education and Research System)
Project ID:BK-05012


