Advantage and Core Benefit
- Mechanism for Reduced Side Effects: As a “G-protein biased agonist,” it selectively activates analgesic signaling pathways. A reduction in side effects such as constipation and respiratory depression has been confirmed in animal studies.
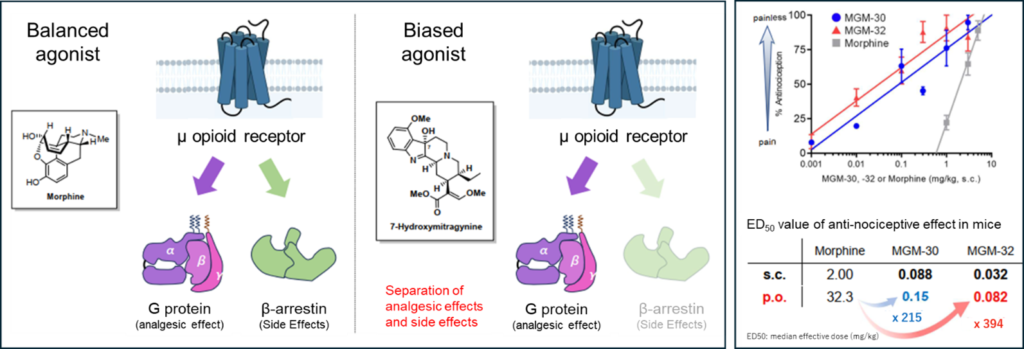
- Oral Administration: Unlike competing drugs that require injection, it exhibits effective analgesic action when administered orally.
- High Metabolic Stability: It demonstrates high stability in human liver microsome assays, suggesting the potential for sustained efficacy in the body.
Background and Technology
Conventional opioid analgesics, such as morphine, provide potent pain relief but present significant clinical challenges due to severe side effects mediated by β-arrestin signaling, including constipation, respiratory depression, and dependence. Through structure-activity relationship (SAR) studies using “7-hydroxymitragynine”—an active compound from the Southeast Asian medicinal plant (Mitragyna speciosa)—as a lead compound, the inventors have created a series of novel compounds, including MGM-30, with dramatically enhanced potency and improved metabolic stability.
 |
Data
- Analgesic Potency: The MGM series of compounds shows high binding affinity for the μ-opioid receptor (MGM-30 Ki value: 0.12 nM). In mouse studies, MGM-30 was confirmed to have 215 times the analgesic potency of morphine, and the subsequent compound MGM-32 showed even greater potency.
- Effects on Intestinal Function and Respiration: In animal studies, at dosage levels that produce an analgesic effect, the constipating effects characteristic of conventional drugs were almost unobserved. Recovery from respiratory depression was also faster than with morphine. However, drug dependence like that of morphine was observed.
- Pharmacokinetics and Other Safety Data: MGM-30 was confirmed to have high metabolic stability in human liver microsomes (92% remaining after 30 minutes). In initial toxicity studies using related compounds (Ames test, hERG assay), no problematic results were found.
Patent & Publication
Patents: Japan: JP 6376702, US: US 9957262 B2,
International Publication: WO 2015/064573
Intellectual Property on new compound (MGM-32): Patent pending
Publications: Journal of Medicinal Chemistry (2002: https://doi.org/10.1021/jm010576e),
Life Sciences (2004: https://doi.org/10.1016/j.lfs.2003.09.054)
Researcher
Dr. Hiromitsu Takayama and Dr. Hayato Ishikawa (Chiba University)
Expectations
To advance this research toward commercialization, we are seeking a partnership with a pharmaceutical company. Specifically, we wish to collaboratively advance GLP-compliant safety studies (toxicology) and detailed pharmacokinetic studies, which are difficult to conduct at a university, to move the project from the preclinical to the clinical development stage. The joint development of a scale-up synthesis process for pharmaceutical manufacturing is also a key area for collaboration. Regarding the form of partnership, we envision a flexible arrangement tailored to the company’s development strategy, including co-research as well as licensing agreements with optional clauses. Further information can be provided under a Non-Disclosure Agreement (NDA).
Project ID:WL-05337


