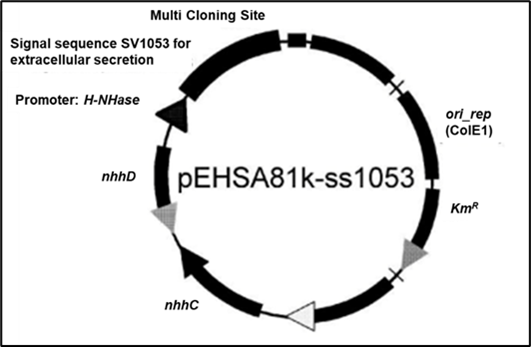
Features and Vector Design
- Designed as a shuttle vector replicable in coli: The plasmid can be constructed in E. coli and subsequently transferred to Streptomyces spp.
- Inducer-free high-level expression: Transcription is activated by the strong promoter system derived from the Rhodococcus nitrile hydratase gene cluster, including the transcriptional regulators NhhC and NhhD.
- Optimized Tat secretion pathway: Efficient extracellular secretion of target proteins is achieved using Tat signal peptides derived from Streptomyces avermitilis.
 |
Background and Technology
To overcome the limitations of protein expression in Streptomyces, we successfully introduced a high-expression system derived from Rhodococcus into Streptomyces hosts. The expression system in Rhodococcus is industrially validated, exhibiting robust transcriptional activity sufficient to produce low-cost commodity chemicals such as acrylamide.
Specifically, we introduced the transcriptional regulators NhhC and NhhD, along with the N-Hase promoter from the Rhodococcus nitrile hydratase gene cluster, into Streptomyces lividans. This demonstrated that the Rhodococcus-based system functions in Streptomyces, leading to the development of a high-expression vector, pHSA81.
To further enable efficient extracellular secretion, we incorporated Tat secretion signal sequences from S. avermitilis and modified the vector to replicate in E. coli, resulting in the construction of a shuttle vector, pEHSA81k-ss1053, optimized for use in Streptomyces. Secretion efficiency was evaluated and confirmed. Additionally, we developed seven other expression vectors containing alternative Tat signal peptides (besides pEHSA81k-ss1053), enabling flexible adaptation depending on the target protein.
Data
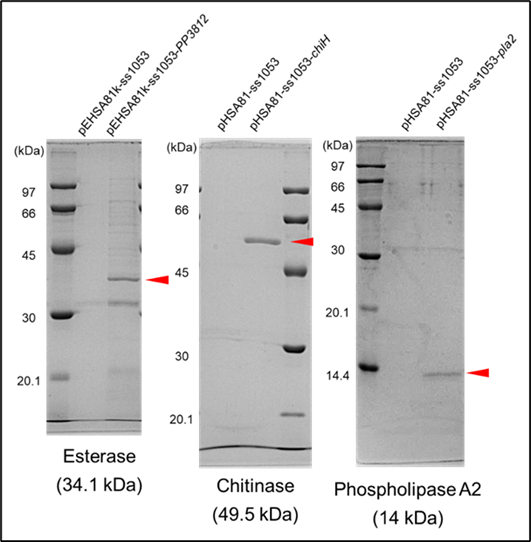
- Successful expression and secretion demonstrated for enzymes such as Pullulanase, Esterase, Chitinase, Phospholipase A2, and Amylase.
- In some cases, the target protein accounted for over two-thirds of the total secreted proteins in the culture supernatant.
 |
Patent
JPB 006906215
Researcher
Dr. Michihiko Kobayashi (University of Tsukuba)
Expectations
We are seeking partners interested in adopting this expression vector system for protein production. Material Transfer Agreements (MTA) for technical evaluation are available.
Project ID:WL-05177


