Advantages
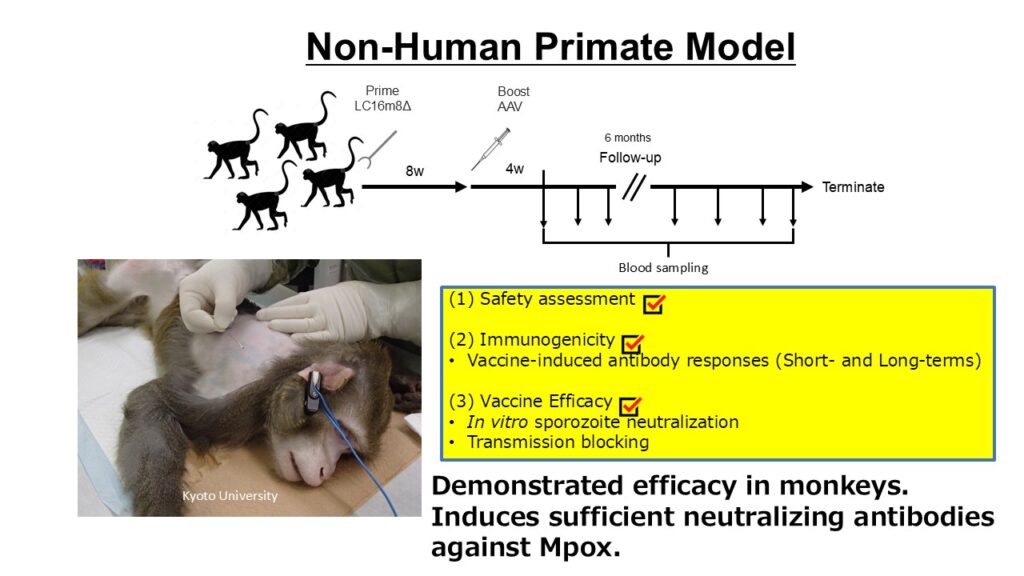
- Concept Proven in Malaria Vaccine: Safety, immunogenicity, and vaccine efficacy confirmed in monkeys; no side effects, strong anti-malaria neutralizing antibodies, and transmission-blocking activity observed. Supported by SCARDA (national center) to advance toward clinical application.
- Versatility for Expansion to Cancer and HIV: A highly adaptable vaccine platform that enables the straightforward development of vaccines for various diseases by simply replacing the antigen gene in the vector.
Technology Overview & Background
Viral vectors are anticipated to deliver antigen genes efficiently to target cells, induce strong immune responses, eliminate the need for adjuvants, and provide prolonged immune activation. Owing to these advantages, they are actively being studied as a vaccine modality for cancer and various infectious diseases.
The conventional malaria vaccine, RTS,S, is a recombinant protein-based vaccine designed to prevent infection. However, in Phase III trials conducted in Africa, it achieved only 30% efficacy, with protection waning after seven years. RTS,S also lacks transmission-blocking capability and requires frozen storage, highlighting the need for innovative vaccine strategies.
Our technology employs a heterologous prime-boost approach, combining two viral vectors with proven safety and immunogenicity: the vaccinia virus strain LC16m8∆ and adeno-associated virus type 1 (AAV1). This combination achieves unprecedented levels of infection prevention and long-term immune persistence.
Specifically, the first (priming) dose with LC16m8∆ induces a potent immune response, while the second (booster) dose with AAV1 ensures long-term antigen expression. This approach allows the platform to avoid the effects of anti-vector neutralizing antibodies, achieving both strong and durable immunity. As such, it leads a novel modality for vaccine development in areas such as infectious diseases and cancer.
In the case of malaria vaccine, we uniquely achieve both infection prevention and transmission blocking, which the conventional RTS,S vaccine could not accomplish. Moreover, it has demonstrated preventive efficacy against emerging infectious diseases such as COVID-19, Zika virus, and monkeypox, expecting its promise as a technology for future pandemic preparedness.
Principal Investigator & Academic Institution
Professor Shigeto YOSHIDA (Kanazawa University School of Pharmacy)
Patents
JP7649504B
Data
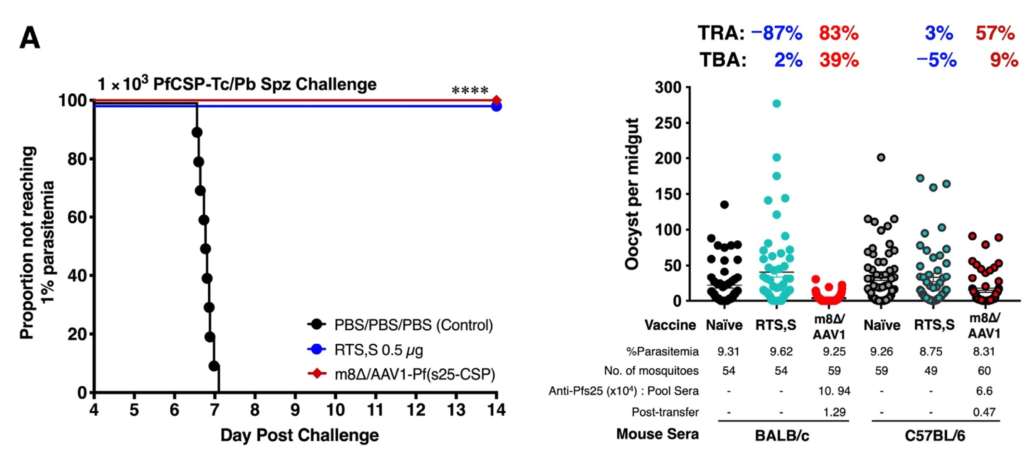
- The LC16m8∆/AAV1 vector carries the pfs25-pfcsp gene, encoding a fusion of the infection-preventing antigen PfCSP and the transmission-blocking antigen Pfs25. In mouse models, two doses of this formulation achieved 100% infection prevention, comparable to three doses of RTS,S (see left figure).
- In transmission-blocking activity (TBA) and transmission-reduction activity (TRA) assessments from mice to mosquitoes, the LC16m8∆/AAV1 formulation demonstrated both effects, whereas RTS,S did not inhibit parasite transmission (see right figure).
- Safety, immunogenicity, and vaccine efficacy have been verified in monkey models.
 |
Collaboration with Company & Future Outlook
This project has been selected for support by the Japan Agency for Medical Research and Development (AMED) under the “Vaccine and New Modality Research and Development Program.” Research and development activities are underway to advance the malaria vaccine toward practical application. We are actively seeking companies interested in collaborating on this program.
We also welcome collaboration with companies interested in utilizing this vaccine platform for indications beyond malaria, as well as proposals for establishing startups.
For further information or to discuss potential collaboration, please contact us.
If you are interested, we would be pleased to arrange an initial online meeting with our research team.
Project ID: TT-05308