Advantage and Core Benefit
- Accurate Evaluation of Challenging Peptides: Enables reliable measurement of low-affinity peptides and hydrophobic peptides that are difficult to assess using conventional methods.
- Cost-Effective and Efficient: Reduces assay costs to 1/5–1/10 compared to traditional synthetic peptide methods.
- Versatile Data Utilization: Generates data usable as training sets for MHCII epitope prediction algorithms, improving prediction accuracy.
Background and Technology
In infectious diseases and tumor immunity, the peptide presentation ability of MHCII plays a crucial role in determining immune responses and serves as a fundamental factor in vaccine design. Particularly in the development of personalized mRNA cancer vaccines, it is essential to identify neoantigens through genomic analysis of tumor tissues and select epitope candidates that can induce immune responses.
In fact, a clinical trial of personalized cancer vaccines targeting renal cell carcinoma patients (Braun et al., Nature 2025) demonstrated that most of the T cells induced after vaccination were CD4+ T cells, highlighting the importance of epitope prediction in MHC class II. However, current epitope prediction tools (such as NetMHCIIpan) face limitations in prediction accuracy, indicating the need for complementary experimental data.
Traditionally, MHCII-peptide binding assays rely on calculating IC50 values using competitive assays with purified MHC proteins. However, the conventional methods face the following challenges:
- Difficulty in measuring hydrophobic or poorly soluble peptides
- Difficulty in evaluating low-affinity peptides
- Limited ability to compare binding across different HLA alleles
- Low reproducibility and quantitatively across different research facilities
To address these challenges, we have developed a novel cell-based assay system that evaluates the binding strength of MHCII-peptide complexes using cultured cells. This system makes it possible to assess the immunogenicity of epitopes and analyze the differences in immunogenicity among HLA alleles, which have been difficult to evaluate using conventional prediction algorithms. Furthermore, this method is expected to accurately assess the impact of cancer neoantigens and viral antigen mutations, areas where existing algorithms are often insufficient.
Data
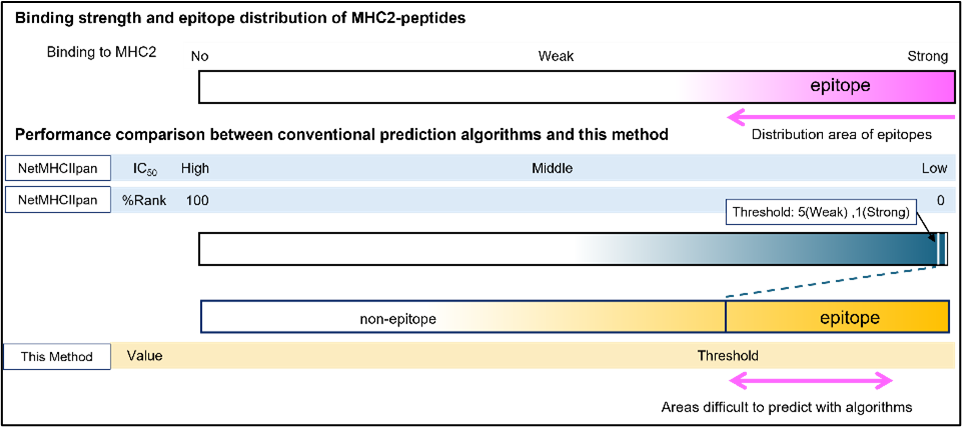
- Comparison of MHCII-Peptide Binding Strength and Epitope Distribution: Conventional epitope prediction methods have a narrow range for strong binding predictions (top 5%) and lack accuracy outside this range. This technology enables the evaluation of epitopes beyond the strong binding range.
- HLA Allele-Specific Differences: Using HLA-DR, HLA-DQ, HLA-DP, and foreign antigens, this technology revealed immune response differences among HLA alleles that were difficult to predict using NetMHCIIpan.
 |
Patent & Publication
Patent pending.
Researcher
Dr. Hiroko Miyadera (University of Tsukuba)
Expectations
We are seeking collaboration with:
- Vaccine developers: To validate the utility of this assay in predicting in vivo immunogenicity, particularly in the context of cancer vaccines and infectious disease vaccines. Retrospective analysis with clinical trial data is also possible.
- Vaccine development CRO/CDMOs: To license this technology and leverage it as part of a proprietary MHC binding prediction solution.
- IT and Data Service Companies: To co-develop automation solutions for large-scale data generation and to enhance MHC binding prediction services using assay-derived data.
Project ID:WL-05107


