Advantages
- Targeting Necroptosis: A novel therapeutic strategy focusing on necroptosis, an inflammatory form of cell death responsible for skin necrosis, and its signaling mechanisms.
- Utilizing an Approved Drug (CDCA): By repurposing chenodeoxycholic acid, an already-approved drug for cholelithiasis, the approach ensures high safety and significantly reduces development risks and costs.
- In Vivo Efficacy of CDCA in SJS/TEN Model Mouse: CDCA significantly suppressed disease onset in a SJS/TEN mouse model transplanted with immune cells from SJS/TEN patients.
Technology Overview & Background
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are severe cutaneous adverse reactions characterized by necrotic lesions in the skin and mucosa. While current treatment relies largely on symptomatic approaches such as systemic corticosteroids, SJS/TEN have high fatality rates (e.g., TEN: 29.9%), highlighting the urgent need for mechanism-based therapies.
The inventors identified necroptosis—in addition to apoptosis—as a key driver of cell death in SJS/TEN. They targeted FPR1 (Formyl Peptide Receptor 1), a receptor involved in necroptotic signaling, and screened compound libraries and known FPR1 inhibitors to identify potential therapeutic candidates. Chenodeoxycholic acid (CDCA) was identified as a selective FPR1 inhibitor that effectively suppressed necroptosis in SJS/TEN model cells.Furthermore, in a SJS/TEN mouse model transplanted with immune cells from SJS/TEN patients and challenged with causative drugs, CDCA administration significantly prevented disease onset, confirming its FPR1-mediated therapeutic effect in vivo.
As CDCA is a bile acid preparation already approved for the treatment of cholelithiasis, its pharmacokinetics and safety profile are well-established. This provides a strong foundation for its efficient clinical application through drug repositioning strategies.
Data
- Necroptosis Inhibition by CDCA in Model Cells: In an in vitro SJS/TEN model cells, which induce necroptosis by adding FPR1 stimulation to immortalized cultured epidermal cells, the cell death inhibitory effect of candidate drugs was evaluated, CDCA exhibited a marked inhibitory effect on cell death (Fig. 1).
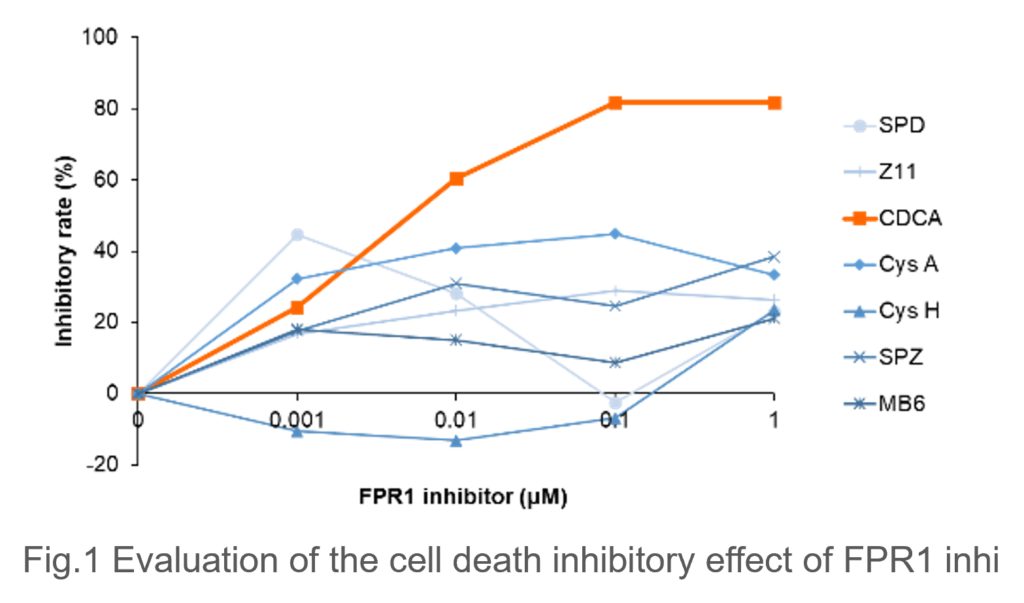 |
- Therapeutic Efficacy of CDCA in Model Mice: In a model where immunodeficient mice were transplanted with immune cells from SJS/TEN patients and administered the causative drug, all mice (7/7) developed blepharoconjunctivitis. However, in the group treated with both the drug and CDCA, disease onset was completely suppressed (0/7) (Fig. 2).
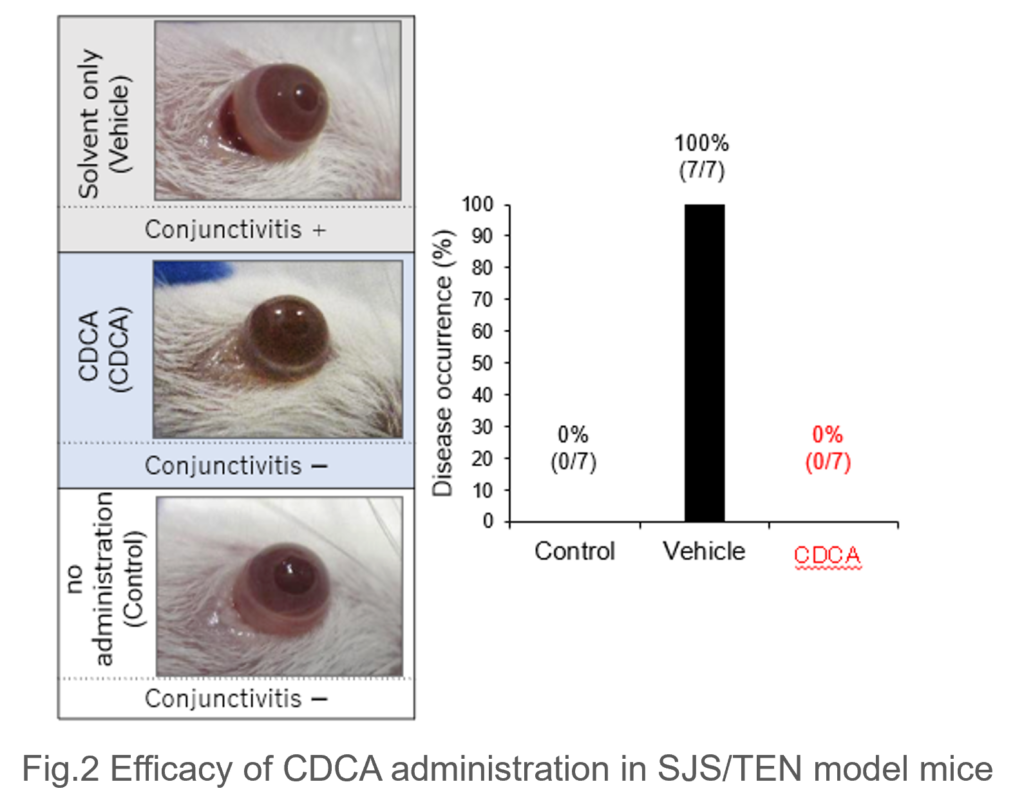 |
Patent
- Patent pending (unpublished)
Researchers & Academic Institution
Prof. Riichiro Abe and Asst Prof. Akito Hasegawa
(Division of Dermatology, Graduate School of Medical and Dental Sciences, Niigata University)
Expectations
Niigata University is seeking corporate partners interested in collaborative research and licensing agreements for the repositioning of CDCA as a new drug. Potential developments include changes to administration routes or dosage forms and re-approval as a novel therapeutic.
Upon execution of a non-disclosure agreement (NDA), we can share unpublished data. We also welcome direct discussions with the inventors. Please feel free to contact us for further information.
Project ID:ML-05287


