Advantages
- Despite being a short-chain peptide comprising fewer than 10 amino acids, it binds specifically to EphA2 and is internalized into cells via receptor-mediated endocytosis.
- Its small size facilitates easy synthesis and quality control, providing substantial cost advantages.
- This peptide is well suited for Theranostics applications, enabling both molecular imaging and nuclear medicine-based therapy.
Technology Overview & Background
EphA2 is a receptor tyrosine kinase located on the cell membrane, known to play a role in cancer progression and malignancy. It is overexpressed in a wide range of cancers, including breast, lung, esophageal, colorectal, cervical, ovarian, and prostate cancers, while showing limited expression in normal tissues. This expression profile makes EphA2 a promising therapeutic target in oncology.
Amid the growing demand for personalized medicine in recent years, the Theranostics approach—where the radioactive isotope used to label compounds that bind to cancer-specific target molecules is modified to enable both diagnosis and treatment—has been gaining increasing attention. Research and development in this field are actively progressing both domestically and internationally. A major advantage of this approach is that using the same compound for both diagnosis and therapy allows for the minimization of side effects and the optimization of therapeutic efficacy.
The inventors’ group has developed Zr-89- and Lu-177-labelled EphA2 antibodies, designed respectively as PET imaging probes and beta-emitting therapeutic agents. The Zr-89-labelled antibody exhibited excellent tumor targeting and accumulation, while the Lu-177-labelled version demonstrated marked tumor regression. These results provide the strong potential of EphA2-targeted Theranostics as a novel and effective cancer treatment strategy. (Watabe T, et al. Eur J Nucl Med Mol Imaging 2025; 52:2887–2897)
On the other hand, the development of RI-labelled antibody therapeutics faces several challenges, including a limited selection of radioisotopes compatible with the biological half-life of antibodies, high manufacturing costs, and complex quality control requirements. Fragmenting antibodies to reduce their molecular size is an emerging solution to overcome these challenges and has attracted considerable attention.
In this study, a peptide derived from the EphA2 antibody was developed. It demonstrated specific accumulation in EphA2-positive cancer cells, effective cellular internalization, and a clear antitumor effect in vivo.
Data
- [²²⁵Ac]-labelled peptide-1 was added to human osteosarcoma cells(HT1080) expressing EphA2, and the level of radioactivity uptake into tumor cells was measured after incubation. The highest uptake was observed at 30 minutes. Compared to the control group (HEK293 cells), a significantly higher level of uptake was seen in HT1080 cells, demonstrating EphA2 selective accumulation in cancer cells.
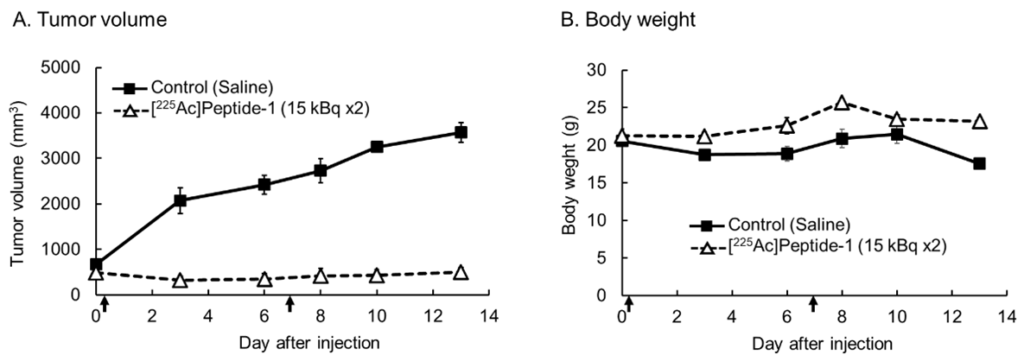
- A twice intravenous administration of [²²⁵Ac]-labelled peptide-1 in osteosarcoma model mice (inoculated with HT-1080 cells), resulted in significant suppression of tumor growth (Figure A), with the therapeutic effect persisting for up to two weeks post-administration. No notable abnormalities in body weight were observed compared to the control group (Figure B), suggesting a favorable safety profile.
 |
Patents
Patent pending (unpublished)
Principal Investigator & Academic Institution
Invited Associate Professor. Yoshifumi SHIRAKAMI (Institute for Radiation Sciences, Osaka University)
Expectations
TECH MANAGE are seeking companies interested in developing diagnostic and therapeutic agents using this peptide. If you are interested, we can provide samples of this fragmented antibody and conduct evaluation tests. Additionally, we can arrange technical meetings with researchers regarding this invention, so please feel free to contact us if you are interested.
Project ID:TT-05212


