Advantages
- Correcting individual differences in tumor markers
- High prognostic prediction ability
- Judgment by one genotype test
- Possibility of expanding the scope of application
 |
Current Stage and Key Data
Research stage using clinical samples.
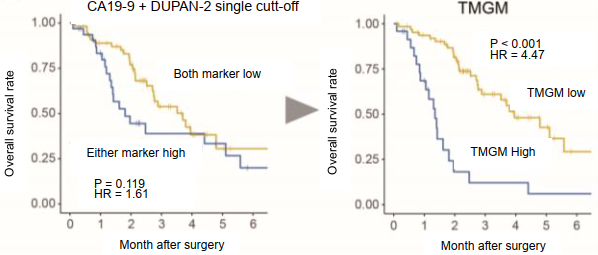
TMGM showed high prognostic predictive ability in patients with unresectable (UR) cases at diagnosis who underwent conversion surgery after medical therapy
 |
Partnering Model
Seeking license partners or collaborative research partners.
- Examples of potential partners: diagnostic companies, SNP diagnostic kit/equipment companies, genetic diagnostic companies, testing centers.
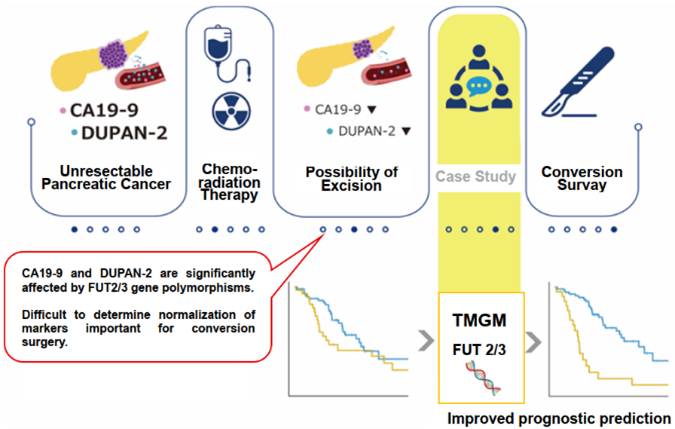
Background
In the pancreatic cancer treatment guidelines, drug therapy is performed according to whether the patient is unresectable (UR), difficult to resectable (BR), or resectable (R), and the timing of resection is determined by changes in tumor markers DUPAN-2 and CA19-9. However, the large individual variability in tumor marker values has been an issue.
FUT2/3 are enzymes involved in the metabolism of Lewis glycans such as DUPAN-2 and CA19-9. Previous reports have suggested that inactivating mutations in FUT2/3 (occurrence rate of approximately 10-20%) affect each marker value and the threshold value as an early diagnostic marker. We have investigated the relationship between tumor marker DUPAN-2 and CA19-9 values, FUT2/3 gene type, resectability classification, and post-resection prognosis, and have developed a tumor marker gene model (TMGM) by combining these as an index for determining the timing of pancreatic cancer resection surgery.
Principal Investigator
Haruyoshi Tanaka (Digestive and Oncological Surgery, Nagoya University Hospital, Tokai National Higher Education and Research System)
Reference
- Article: Tanaka et al., Br J Surg. (2025) 112(4):znaf049.
- Patent: Patent pending (unpublished) *Additional information may be disclosed under CDA.
Project ID:BK-05072


