Advantages
- It has higher diagnostic performance than conventional lung cancer markers (CEA, CYFRA).
- It has a synergistic effect when combined with conventional markers.
- It has particularly high diagnostic performance for early stage (stage I) lung cancer.
Current Stage and Key Data
High diagnostic performance has been confirmed through clinical cohort studies using our proprietary ultrasensitive digital extracellular vesicle screening technique.
- ROC curve AUC value for early stage (stage I) lung cancer diagnosis.
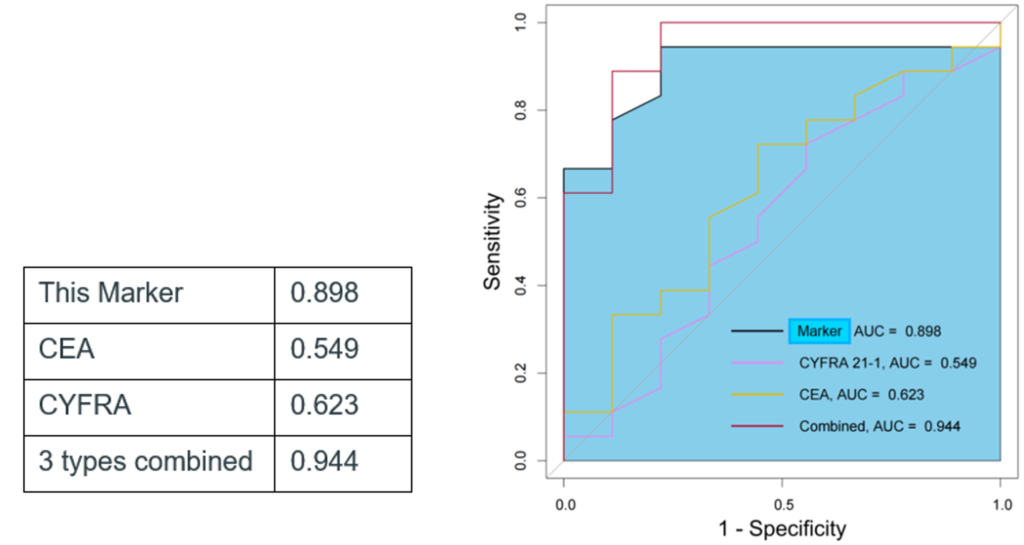 |
Partnering Model
Joint development of the optimization of monoclonal antibodies and ELISA for this marker protein.
Joint research, development and licensing of contract testing services and in vitro diagnostic reagents using this marker.
- Examples of potential partners: antibody development companies, test kit development companies, in vitro diagnostic drug development companies, clinical testing service companies
Background
We collected blood samples from pre- and post-operative lung cancer patients (706 and 428 cases), 22 tumors and normal tissues, blood samples from patients with benign diseases (COPD and interstitial pneumonia) (several dozen cases), and blood from healthy individuals. In this study, EVs were collected from lung cancer and normal lung tissues and subjected to proteomic analysis by LC-MS/MS. The proteomics data were compared with TCGA data to narrow down the candidates, and specific proteins were identified as new biomarkers through literature validation. Their usefulness was validated using plasma samples from lung cancer patients.
Principal Investigator
Taketo Kato (Department of Thoracic Surgery, Nagoya University Graduate School of Medicine, Tokai National Higher Education and Research System)
Reference
- Paper: In preparation for submission.
- Patent: Patent pending (unpublished)
*Additional information may be disclosed under the CDA.
Project ID:BK-05036


