Advantages
- Oral administration possible.
- High safety.
- Improvement of some indicators confirmed in investigator-initiated Phase 2 clinical trial.
Current Stage and Key Data
Investigator-initiated clinical trial Phase 2
- Safety has been tested in investigator-initiated clinical trial Phase 1.
- Improvement in some indicators has been confirmed in Phase 2 investigator-initiated clinical trial (multicenter open-label, 9 patients, meclizine administered once daily for 26 weeks). Detailed data can be disclosed under CDA.
- Efficacy, PK/PD, and safety have been confirmed in animal experiments (see references).
 |
Partnering Model
Seeking partner companies to collaborate on further clinical development and approval applications.
- Examples of potential partners: Biotech/pharmaceutical companies focusing on repositioning and development of treatments for orthopedics and rare diseases.
Background
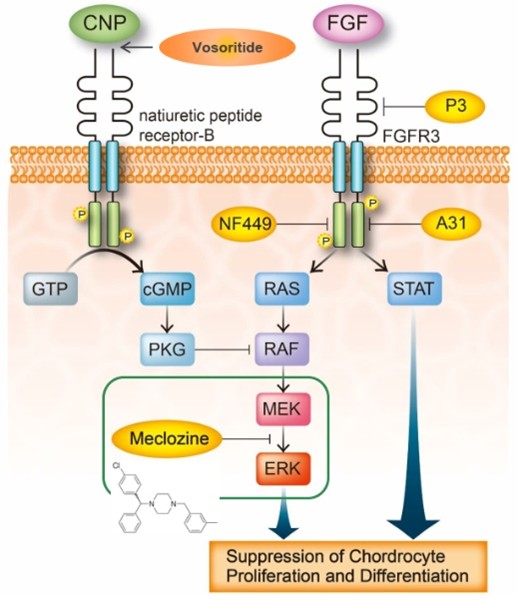
Achondroplasia is a congenital disease that causes short limbs and short stature (final height: 120-130 cm), with a prevalence of about 5 cases per 100,000. It is caused by enhanced signaling due to a gain-of-function mutation in FGFR3. The current standard treatment is growth hormone and limb lengthening surgery, but these are not effective enough. In recent years, vosoritide has been approved as a new therapeutic drug that suppresses FGFR3 signaling (peptide formulation, daily subcutaneous injection). However, the burden on patients remains large, and new therapeutic drugs are needed.
Principal Investigator
Masaki MATSUSHITA (Department of Orthopedics/Rheumatology, Nagoya University Graduate School of Medicine, Tokai Higher Education and Research System)
References
- Papers: Matsushita et al. (2017) Sci Rep. 7;7(1):7371
- Patents: JP Patent No. 6232630
Project ID:BK-03867


