Advantages
- The therapeutic drug is also suitable for infants.
- Low side effects.
- Oral administration.
Current Stage and Key Data
Preclinical research stage
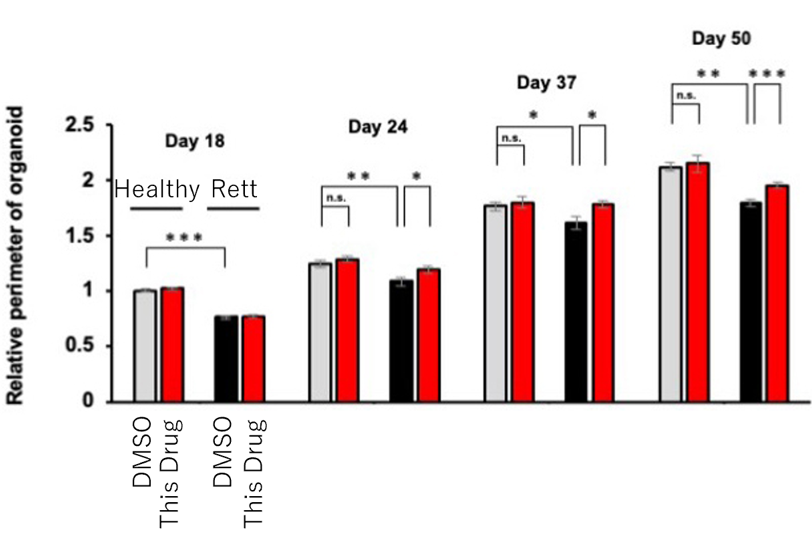
- The therapeutic drug improved neurite outgrowth in Rett syndrome model neurons, promoted the size and neurite outgrowth of neurons derived from patient iPS cells, and restored the size of brain organoids. (in vitro)
- In a rotarod test using model mice, the treated group showed motor coordination equivalent to that of wild-type mice. (in vivo)
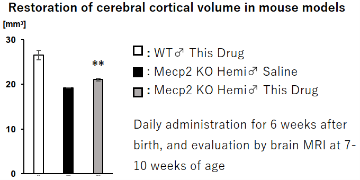
- The treated group showed a recovery from the reduction in brain volume compared to the non-treated group. (in vivo)
- Behavioral toxicity showed a decrease in social and motivated behavior, but no other abnormalities were observed. (in vivo)
 |
 |
Partnering Model
Seeking an exclusive license partner for this patented technology.
- Examples of potential partners: Biotech/Pharma companies focused on Rett Syndrome, rare diseases, and/or repositioning drug development.
Background
Rett syndrome is a progressive neurodevelopmental disorder caused by a mutation in the MECP2 gene (designated an intractable disease). It occurs in 1 out of every 10,000 to 15,000 girls (boys die prenatally), and there are approximately 64,000 patients worldwide. The child develops normally until about 18 months of age, but then loses motor and language skills. Clinical symptoms include autism spectrum disorder, epilepsy, intellectual disability, microcephaly, motor dysfunction, respiratory disorders, and general growth retardation. Trofinetide (IGF-1 analog) was approved by the US FDA in 2023 as a treatment, but the improvement in clinical symptoms is very slight, and gastrointestinal side effects are a problem. In addition, gene therapy to replenish normal MECP2 genes is being researched, but has not yet been put to practical use. Therefore, new treatments are needed.
Principal Investigators
Ayato Sato (Institute of Transformative Bio-Molecules (ITbM), Nagoya University, Tokai National Higher Education and Research System)
Satoru Takahashi (Department of Pediatrics, Asahikawa Medical University)
Yukihiro Noda (Faculty of Pharmacy, Meijo University)
Reference
- Paper: Unpublished. Patent: Pending (unpublished).
- CDA allows disclosure of the therapeutic drug and detailed data.
Project ID:BK-05257


