Advantages
- Elongation of two residues at a time in a short time with high yield.
- High purity is achieved by recrystallization without column purification.
- Raw materials and reactants are inexpensive and do not require excessive amounts.
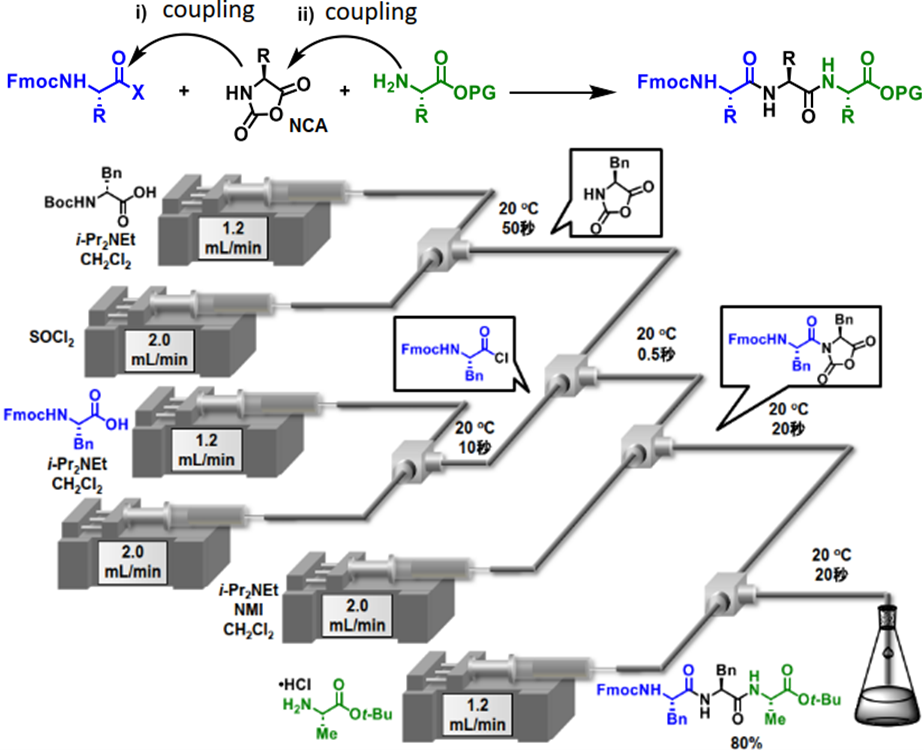 |
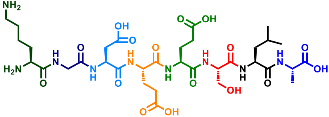
Current Stage and Key Data
- Various tripeptides have been synthesized, and high yields have been confirmed.
 |
|
 |
Partnering Model
- Seeking joint development partners for peptide pharmaceuticals, cosmetics, food, and agrochemicals using this synthesis method, as well as license partners for this synthesis method.
- Examples of potential partners: CMC divisions of biotech/pharmaceutical, cosmetics, food, or agrochemical companies that develop and manufacture peptide materials, CMOs/CDMOs, and chemical companies that focus on flow synthesis.
Background
In recent years, peptides have become increasingly important as medium molecule drugs, and since approximately 85% of marketed drugs are supplied by chemical synthesis, there is a growing need for highly efficient manufacturing processes. However, the synthesis cost of peptide drugs is about 10-100 times higher than that of small molecule drugs. The most common synthesis method for peptides involves repeated linking of protected amino acids and removal of protecting groups, which increases costs, waste, and the labor and time required for synthesis. It would be ideal if amino acids could be elongated consecutively two residues at a time without a deprotection step, but the previously reported methods had problems such as long-term conditions, expensive reactants, and the need for excessive amounts of raw materials.
Principal Investigator
Shinichiro Fuse (Department of Basic Medicine Science, Graduate School of Pharmaceutical Sciences, Nagoya University, Tokai National Higher Education and Research System)
Reference
- Papers: Sugisawa et al., Chem. Sci. (2023) 14, 6986-6991
- Patents: WO2023/223714 (JP, US, EP, CN, IN) pending
Project ID:BK-04932


