Advantages
- Functional IgE Receptors for Allergy Research: cSIMVA1 expresses functional IgE receptors, enabling allergy response studies, degranulation assays, and desensitization experiments.
- Cytokine-Free, Cost-Effective Cultivation: Grows without IL-3 or SCF, reducing costs and simplifying long-term maintenance.
- High-Throughput Screening for Drug Discovery: Supports efficient screening of low- and medium-molecular-weight compounds for vaccines, therapeutics, and allergy diagnostics.
Technology Overview & Background
Mast cells play a central role in allergic reactions, making their activity assessment crucial not only for vaccine and drug development but also for allergy testing related to food and chemicals. Traditional mast cell evaluation systems often use mouse bone marrow-derived cells, but their induction requires significant time, and their proliferation and maintenance depend on cytokines such as IL-3 and SCF, which poses a challenge. Moreover, although several human and mouse mast cell lines have been established, they lack functional IgE receptors, and none-IgE receptors, making them inadequate for allergy evaluation.
Dr. Kurashima discovered a highly proliferative mast cell line named cSIMVA1 (Cell-line of Serosa-Infiltrated Mast Cell for Versatile Analysis 1) after long-term co-culture of mouse mast cells and support cells.
- cSIMVA1 proliferates independently of cytokines such as IL-3 and stem cell factor (SCF) and has been confirmed to function in mucosal and epidermal environments within the body.
- Additionally, cSIMVA1 expresses IgE receptors, making it suitable for allergy research, degranulation experiments, and even desensitization studies that simulate immunotherapy.
- This cell line is expected to serve as a high-throughput and user-friendly allergy reaction evaluation system.
Data
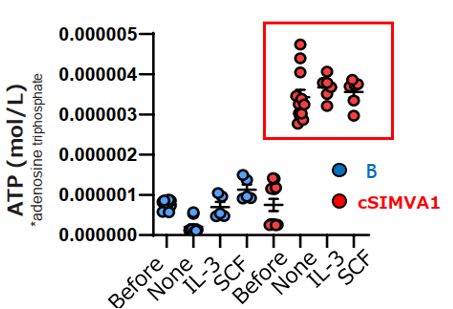
- Mouse bone marrow-derived mast cells (BMMC) and cSIMVA1, intracellular ATP concentration (indicative of proliferation rate) was measured under control conditions (None) and with the addition of IL-3 and SCF. In all groups, cSIMVA1 showed increased ATP concentration, confirming its proliferation. (Before represents ATP concentration before the experiment.)
 |
Principal Investigator
Dr. Yosuke Kurashima (Chiba University)
Expectations
Chiba University is looking for companies interested in the following applications of this technology:
- Seeking to conduct library screening for anti-allergy drugs
- Evaluating allergies and anaphylactic reactions related to food and therapeutic modalities
- Aiming to identify novel factors related to allergies and pseudo-allergies
- Providing contract services for allergy effect evaluation tests
Collaboration opportunities are available for research and drug development utilizing the cSIMVA1 evaluation system, as well as for paid evaluations of the cell line.
Project ID: WL-05128


