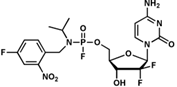
Advantages
- Low cytotoxicity.
- Controlled deprotection in cells.
- A platform technology that can be applied to various nucleic acid analogs.
Current Stage and Key Data
- The fluoride-type phosphate prodrug was taken up into cells and monophosphorylated. Furthermore, deprotection did not cause toxicity to normal cells.
- In an in vitro experiment using a gemcitabine-resistant pancreatic cancer cell line, the gemcitabine prodrug of the present invention showed a higher anticancer effect than existing ProTide-type prodrugs.
Partnering Model
- Joint research and development to design and evaluate fluoride-type phosphate prodrugs of nucleic acid analogs at the request of partner companies.
- Examples of potential partner companies: biotech companies, pharmaceutical companies, CDMOs, etc.
Background
Nucleic acid analogs are used as metabolic antagonists, such as anticancer drugs (gemcitabine, cytarabine, etc.) and antiviral drugs (lamivudine, zidovudine, etc.). These are monophosphorylated in cells and then converted to triphosphates (active forms), which inhibit the synthesis of DNA and RNA necessary for the proliferation of cancer cells and viruses. However, target cells develop resistance mechanisms such as inhibition of intracellular translocation of nucleic acid analogs, inhibition of intracellular phosphorylation, and promotion of degradation. In order to avoid such resistance, various phosphate prodrugs such as BisPOM type and ProTide type have been developed, but problems include the release of toxic substances during deprotection and limited deprotection conditions.
The present invention is a novel fluoride-type phosphate prodrug compound that has low cytotoxicity and can control deprotection in cells.
Principal Investigator
Hiroshi Abe (Department of Chemistry, Graduate School of Nagoya University, Tokai National Higher Education and Research System)
Reference
- Yoshida et al., ChemMedChem (2022)17, e202200188 (1 of 5)
- Patent Application JPA-2022-117326 (pending)
Project ID: BK-04901