Advantages
- Easily create a mouse model of infection with human-infectious pathogenic Enterobacteriaceae bacteria.
Current Stage and Key Data
- At the stage where it can be put to practical use for research and evaluation.
- An O157-infected mouse model has been created, and Shiga toxin production and hemolytic uremic syndrome symptoms have been confirmed (Figure).
- Similar results have been obtained with mice of different developmental backgrounds and multiple types of pathogenic E. coli.
- It has been confirmed that the therapeutic effects of fecal transplants and antibiotics can be verified using this model.
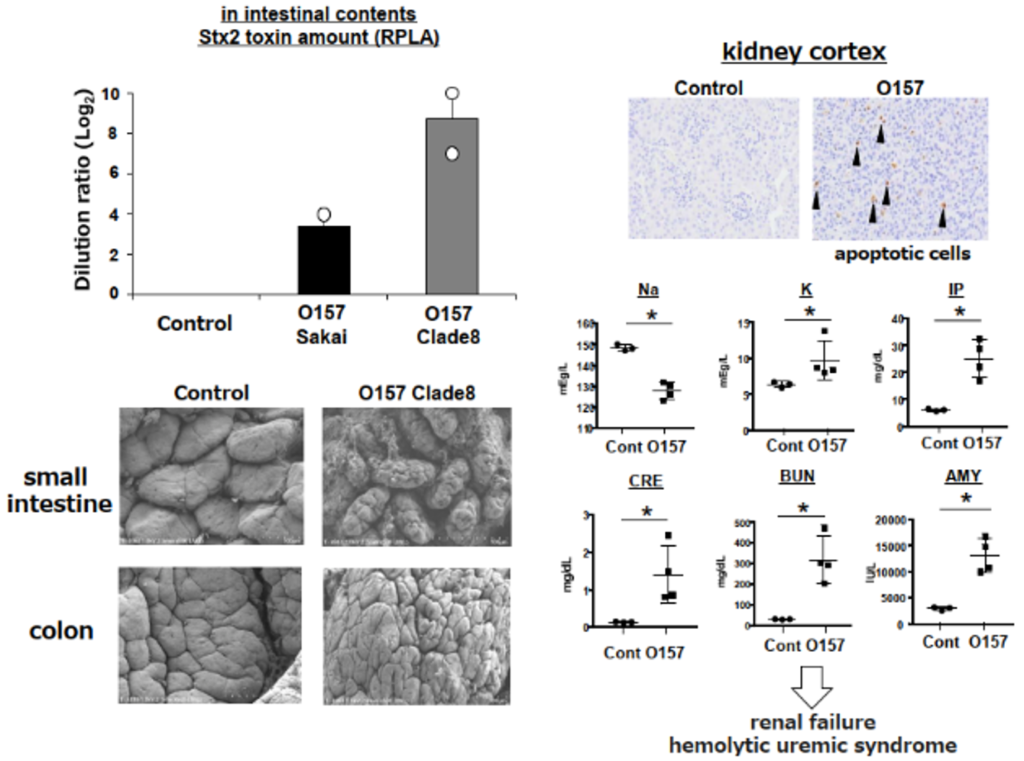 |
Partnering Model
Patent license for the infection model mouse and its production kit using this technology.
Examples of potential partners:
- Drug discovery companies/CROs that use model mice for non-clinical research and development of O157 antibacterial agents and toxin neutralizers
- Food and hygiene-related companies that use model mice for food testing, etc.
- Research reagent companies and experimental animal supply companies that provide model mice and their production kits to researchers.
Background and Technology
Infection with Enterobacteriaceae bacteria causes many types of intestinal infections. For example, enterohemorrhagic E. coli O157, a pathogenic E. coli, has caused many outbreaks around the world in the past. Therefore, methods for treating and preventing intestinal infections are required.
However, human-infectious Enterobacteriaceae bacteria do not easily colonize the intestines of non-human animals such as mice, and there have been no suitable animal models for infection. In many basic studies, mouse infection models using Salmonella Typhimurium (mouse typhoid bacteria) or Citrobacter rodentium (mouse pathogenic E. coli) as mouse infectious pathogens are used. In addition, a human Enterobacteriaceae infection model using germ-free mice is known, but it is not practical because it requires a lot of effort and cost to create. Therefore, the in vivo bacterial infection mechanism and host defense response have not been elucidated, which is an obstacle to the development of new drugs.
Principal Investigator
Yoshiyuki Goto (Medical Mycology Research Center (MMRC), Chiba University)
Reference
Patent pending (unpublished yet)
Project No.BK-04976


