Advantage and Core Benefit
- Targeted Gene Expression: Utilizes a synthetic receptor system that induces gene expression specifically in disease tissues in response to inflammatory environments.
- Validated in vivo: Produces anti-inflammatory factors and growth factors by the designer cells at ulcerated sites in mouse models of colitis.
Background and Technology
Recent advancements in stem cell research have enabled organoid culture systems that replicate parts of organs ex vivo, opening the door for regenerative medicine using stem cell and organoid transplantation. However, tissue regeneration in chronic inflammatory regions, where essential microenvironmental niches are lost, remains challenging. The use of potent anti-inflammatory or growth factors for tissue regeneration raises concerns about side effects on healthy tissues.
In inflammatory bowel disease (IBD), even when inflammation is partially controlled with anti-inflammatory drugs, refractory ulcers may form, preventing epithelial regeneration. Some patients are left with no treatment options other than colectomy.
To address this, the inventors developed “designer niche cells” to reconstruct niches specifically in affected regions, using IBD as a model disease. These cells leverage the synthetic Notch receptor (synNotch) system to recognize tissue environmental changes, such as inflammation or ulcers, and produce anti-inflammatory and growth factors accordingly. Validation in vitro and in vivo using colitis mouse models confirmed that these cells promote intestinal organoid growth and engraft at ulcer sites. This technology holds promise as a foundational approach for cellular therapeutics targeting intractable inflammatory diseases like IBD.
Data
In vitro validation of designer niche cells:
Co-culture of intestinal organoids secreting GFP (a green fluorescent protein) with designer niche cells that recognize GFP resulted in activation of the niche cells near the organoids. Even in the absence of external niche factors, the activated designer niche cells promoted the growth of intestinal organoids.
Designer niche cells producing anti-inflammatory factors were shown to suppress the production of inflammatory cytokines by macrophages stimulated with LPS.
Validation in colitis mouse models:
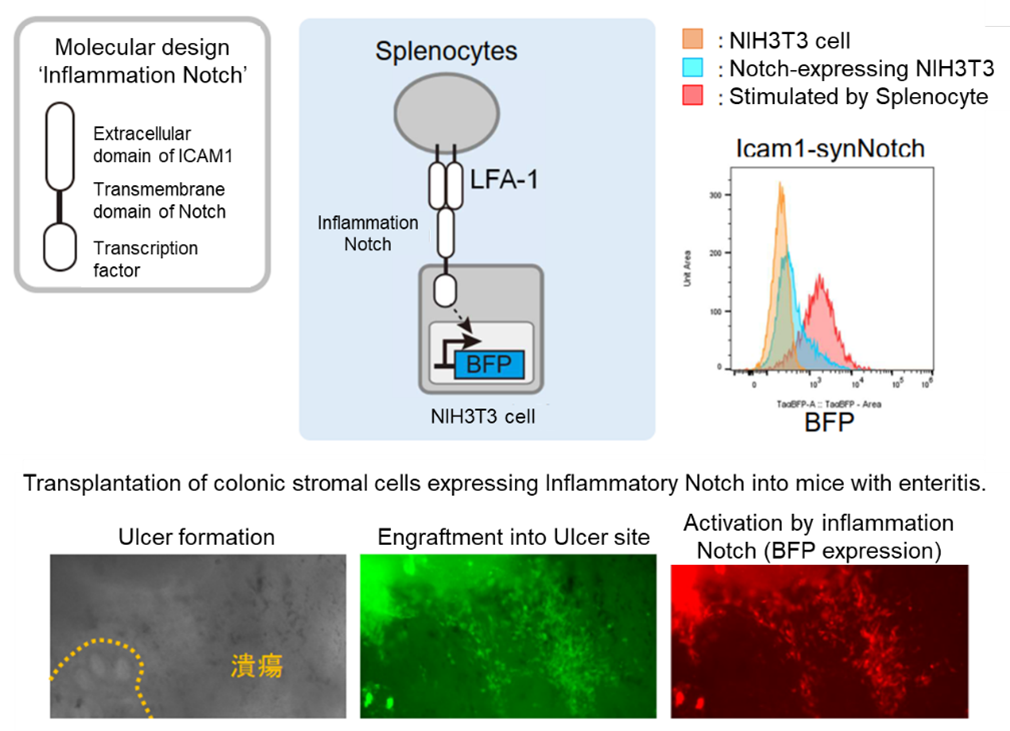
Designer niche cells, derived from colonic stromal cells and engineered with synthetic receptors to recognize inflammatory cells, were transplanted into the colon lumen of DSS-induced colitis mouse models with ulcerated colons.
These cells engrafted at the ulcer sites, activated their receptors in response to inflammatory cells, and induced the expression of a fluorescent reporter. The cells persisted at the ulcer sites for over five days and were shown to produce anti-inflammatory and growth factors.
 |
Patent & Publication
- Patent: Pending (unpublished).
Researcher
Dr. Satoshi Toda (The University of Osaka)
Expectations
The University of Osaka seeks partner companies interested in developing cell therapies for inflammatory bowel disease using this technology. Meetings with the inventors can be arranged for further discussions.
Project No.WL-05114


