Advantages
- The first therapeutic/preventive agent targeting hepatocarcinogenesis in patients with increased sinusoidal pressure, such as post-Fontan surgery or cirrhosis after hepatitis quiescence.
- CTGF is also a biomarker reflecting sinusoidal intracavitary pressure and has potential for companion diagnostic applications.
Technology Overview & Background
In chronic viral hepatitis and cirrhosis, the mechanism by which fibrosis progresses from hepatocyte death to hepatocarcinogenesis has been elucidated, and it is now possible to suppress liver pathology by inhibiting hepatocyte death. However, there are cases in which liver pathology worsens even when hepatocyte death is suppressed, and although the mechanism and cause of this is unknown, it is known that sinusoidal intracavitary pressure is increased. Congestive liver injury such as Fontan-associated liver disease and Budd‒Chiari syndrome is also known to cause liver fibrosis and hepatocarcinogenesis due to increased sinusoidal pressure, although liver inflammation is not observed.
The researchers focused on liver sinusoidal endothelial cells (LSECs), which are directly exposed to physical stimuli due to increased sinusoidal pressure. As a result of their efforts to elucidate the pathogenic mechanism of liver carcinogenesis caused by increased sinusoidal pressure, they found that connective tissue growth factor (CTGF) is deeply involved in liver carcinogenesis caused by increased sinusoidal pressure. It has long been known that CTGF is involved in development of liver fibrosis and proliferation of hepatocarcinoma cells, but this is the first time that it has been shown to be involved in hepatocarcinogenesis itself. The mechanism is that pressure stimulation activates YAP/TAZ in LSECs via integrin αV on the cell surface, which increases CTGF expression, resulting in LSEC capillarization and enhanced production of fibrosis-related proteins in hepatic stellate cells, leading to liver fibrosis, portal hypertension and hepatocarcinogenesis. As Inhibiting CTGF function also suppresses liver tumorigenesis, CTGF or integrin αV inhibitors are expected to be useful drug candidates for the treatment and prevention of liver carcinogenesis.
In addition, no biomarkers have been established for hepatocarcinogenesis caused by increased sinusoidal pressure, and a search for biomarkers has been desired clinically. In the present study, the inventors identified secret proteins whose expression increased in liver tissue and serum in response to increased sinusoidal pressure, including CTGF. Therefore, CTGF is expected to be used as a biomarker for liver carcinogenesis and as a companion diagnostic marker for treatment/prevention.
Data
- A single-cell analysis of a mouse model of congestive hepatopathy, partial inferior vena cava ligation (pIVCL), showed that YAP/TAZ were activated and that the most upregulated gene was CTGF in zones 2 and 3 LSECs in the pIVCL-treated group compared to the control group.
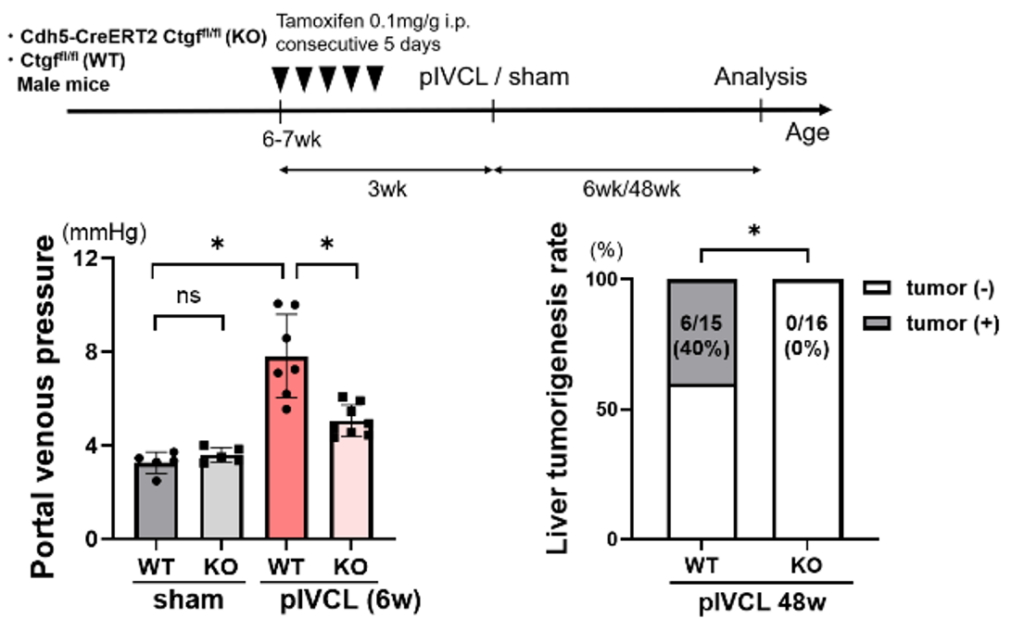
- Tamoxifen-inducible endothelial cell-specific CTGF knockout mice were generated, followed by pIVCL. CTGF knockout in LSECs suppressed pIVCL-induced liver fibrosis, portal hypertension and liver tumorigenesis caused by hepatic congestion.
 |
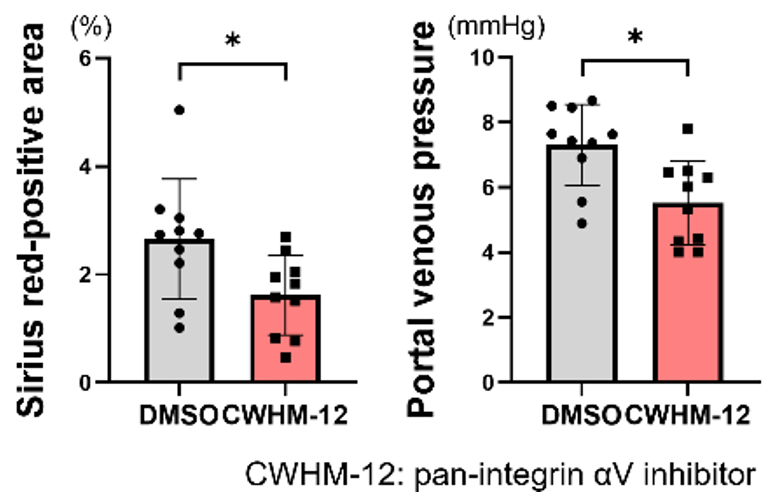
- The pan-integrin αV inhibitor CWHM-12 (100 mg/kg/day) was administered to mice by continuous subcutaneous injection beginning 2 weeks after pIVCL. Integrin αV inhibition also alleviated pIVCL-induced liver fibrosis and portal hypertension with decreased CTGF expression.
 |
Patent(s)
A Japanese patent has been filed and a PCT application is being prepared.
Principal Investigator & Academic Institution
Hayato HIKITA, MD, PhD (Associate professor, Graduate School of Medicine, The University of Osaka)
Expectations
TECH MANAGE is now looking for pharmaceutical/diagnostic companies that are interested in developing and commercializing products based on this invention.
We can also arrange a direct meeting with the principal investigator on this topic. In addition, we can provide unpublished data and know-how through the conclusion of a CDA with The University of Osaka. We can also set up options such as exclusive evaluation before licensing the invention, so please feel free to contact us.
Project No.JT-04939


