Advantages
- Low environmental impact reactions; no high pressure, low temperature reactions (about 25 °C) and no deuterium gas
- High reaction performance(80-99% introduction, 80-98% yield)
- Proven deuteration for ‘Ibuprofen’ and can also be used for a wide range of compounds, post-synthesis and on-demand synthesis
- Raw material, deuterated water (D2O) is reusable and easily purified from the catalyst
Background & Technology
As deuteration of pharmaceuticals is expected to improve drug stability, improve pharmacokinetics and reduce toxicity, there is a move towards the strategic development of deuterated pharmaceuticals, utilizing the benefits of this process. On the other hand, all conventional deuteration processes are batch-type deuteration methods using organic synthesis processes, and the complexity and cost of treatment methods and processes have been an issue.
The laboratory has developed a deuterium-labelled compound flow synthesis system that can be processed at ambient pressure and room temperature, using a process that does not involve organic synthesis and does not produce a large amount of waste and demonstrated deuterated pharmaceutical.
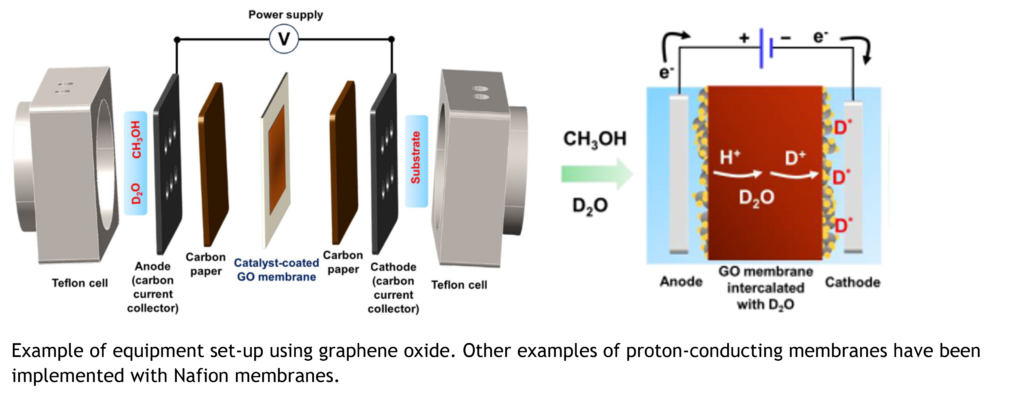
To be specific, the device concept is to supply heavy water (D2O) and a proton source to the anode side of the proton-conducting membrane and a compound to be deuterated to the cathode side of the proton-conducting membrane by means of a new membrane-type reactor with electrodes attached to the proton-conducting membrane. Nafion and graphene oxide membranes are currently used as proton-electric membranes. Deuterium-labelled compounds are electrochemically synthesized by energizing a proton-conducting membrane, whereby the proton source and heavy water are electrochemically oxidized as H+/D+ on the anode side and react with the deuterated compound as D+/D2 that passes to the cathode side via the proton-conducting membrane.
The system can contribute to the research and development/manufacturing stages of a wide range of pharmaceuticals, allowing high-performance pharmaceuticals to be considered.
 |
Researcher
Kumamoto University Department of Applied Chemistry and Biochemistry Professor Testuya Kida
Patent
Pending(International phase, unpublished)
Publication
https://doi.org/10.1021/acs.nanolett.3c04243
Development Phase
Current stage:
We have obtained a POC for a deuterated system with prototype equipment. Implementation cases and mechanism verification ongoing.
Next stage:
i) Evaluation of the versatility of deuterated pharmaceuticals, evaluation of pharmaceutical development.
ii) Catalyst development, optimization and application development for further reaction efficiency.
iii) Commercialization of the device through the development of mass production equipment.
All of the above can be done in collaboration/joint development with research laboratories. We are looking for partner companies interested in evaluating sample pharmaceuticals using this technology/process, joint research/development collaboration and technical guidance. We would be happy to start with a detailed description and discussion of the technology.
Project No.ON-04951


