Advantage and Core Benefit
- The artificial nucleic acids enable direct administration of naked siRNA into the CNS, improving tissue distribution and pharmacological effects
Background and Technology
Polyglutamate diseases are intractable neurological diseases caused by genes harboring CAG repeat expansions and include spinal and bulbar muscular atrophy (SBMA), Huntington’s disease and spinocerebellar ataxias. In SBMA, expanded CAG repeats in the androgen receptor (AR) gene lead to the accumulation of abnormal AR proteins, and cause muscle wasting and dysphagia. Nucleic acid medicines targeting CAG repeat are expected to be applicable broadly to polyglutamine diseases. However, there is concern that genes with normal CAG repeats may be concurrently inhibited, which would compromises physiological functions in the CNS.
We have confirmed that siRNAs with an unlocked nucleic acid in the sequence of siRNA targeting CAG repeats can selectively suppress the expression of abnormal polyglutamine proteins. However, the siRNA was required to be encapsulated in lipid nanoparticles as in vivo drug delivery system. In this study, siRNA targeting CAG repeats was developed with use of an innovative artificial nucleic acid, and when this siRNA acted on normal or SBMA patient-derived fibroblasts, it suppressed the expression of mutant AR protein without affecting the expression of its normal counterpart. Furthermore, intracerebroventricular administration of the naked siRNA in SBMA model mice exerted a knockdown effect of mutant AR distally in the spinal cord and also an improvement in motor function.
Data
- When the siRNA acted on normal fibroblasts (AR with 18 polyglutamines [AR-18Q]) and fibroblasts from SBMA patients (AR-54Q), it selectively suppressed the expression of mutant AR protein.
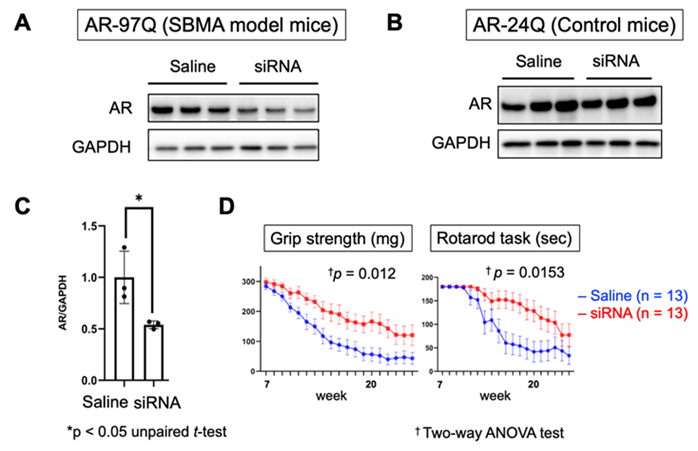
- When the siRNA was intracerebroventricularly administered in SBMA model mice (AR-97Q), knockdown of the mutant AR was confirmed in the spinal cord 7 days post administration (A, C), and an improvement in motor function was achieved (D). In contrast, the siRNA had no effect on AR expression in control mice harboring AR with a normal CAG repeat (AR-24Q) (B).
 |
Patent
Patent Pending (Unpublished)
Expectations
We are interested in partnering with companies that would be willing to license the invention and co-develop nucleic acid medicines for polyglutamine diseases. We are also interested in collaborating with companies that develop nucleic-acid technologies to extend the duration of action of nucleic acids as well as their application to other polyglutamine diseases including Huntington’s disease and spinocerebellar ataxias.
Project.WL-04890


