Advantages
- Materials with high-strength double network hydrogel (DN gel) developed in this laboratory
- Flexibility, high toughness, and strong bonding due to spontaneous bone penetration
- Proven implantation in bone defects of rabbit femoral condyle
- Available to provide samples, advice on manufacturing and molding techniques
Background & Technology
Damaged and worn knee cartilage and joints do not heal spontaneously in vivo and require treatment. The market for knee cartilage defect repair is expanding significantly due to the increasing number of knee injuries and the widespread use of minimally invasive surgeries. For cartilage defects up to a certain size, techniques such as “mosaic plasty” and “autologous cultured cartilage transplantation,” in which normal cartilage is collected and transplanted to the damaged area, are generally applied. However, these treatments are not applicable to large injuries because they require the collecting of normal cartilage of the same size as the repaired area or involve two surgeries, one for harvesting and the other for transplantation, which places a heavy burden on the patient.
In order to solve these problems, the laboratory has developed an original “high-strength DN gel” and has been conducting research to use it as artificial cartilage with the same level of toughness and water content as biological cartilage and a surface with high sliding properties comparable to biological cartilage. Finally, this laboratory found a way to complex HAp particles, the main inorganic component of bone, with the surface layer of DN-gel, and succeeded in establishing a method to bind a strong and flexible hydrogel to bone in vivo.
With the implantation of this material, the treatment of cartilage defects will eliminate the need for donor extraction of normal cartilage, providing a better treatment option for the elderly and patients who wish to avoid the burden of surgery.
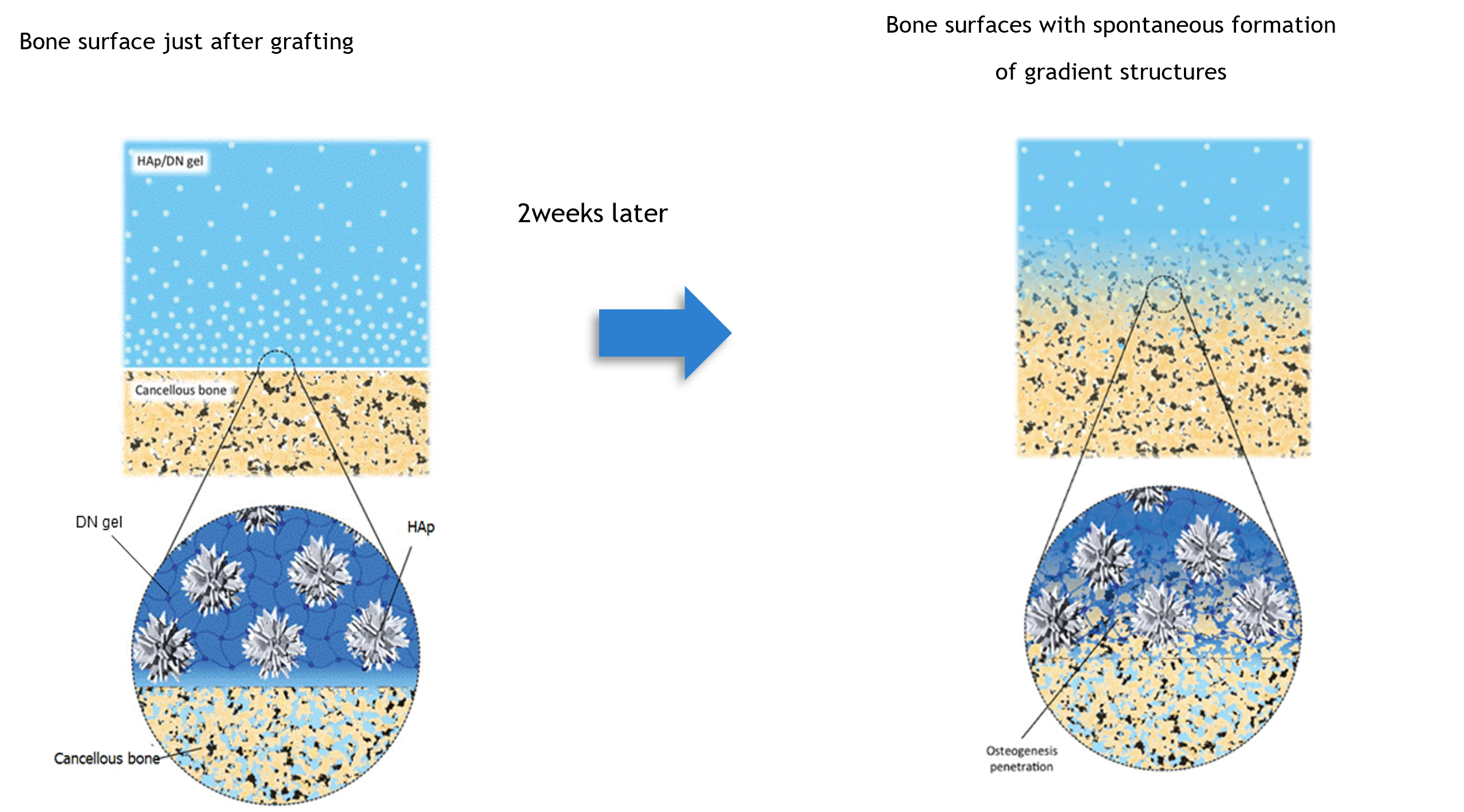 |
Data
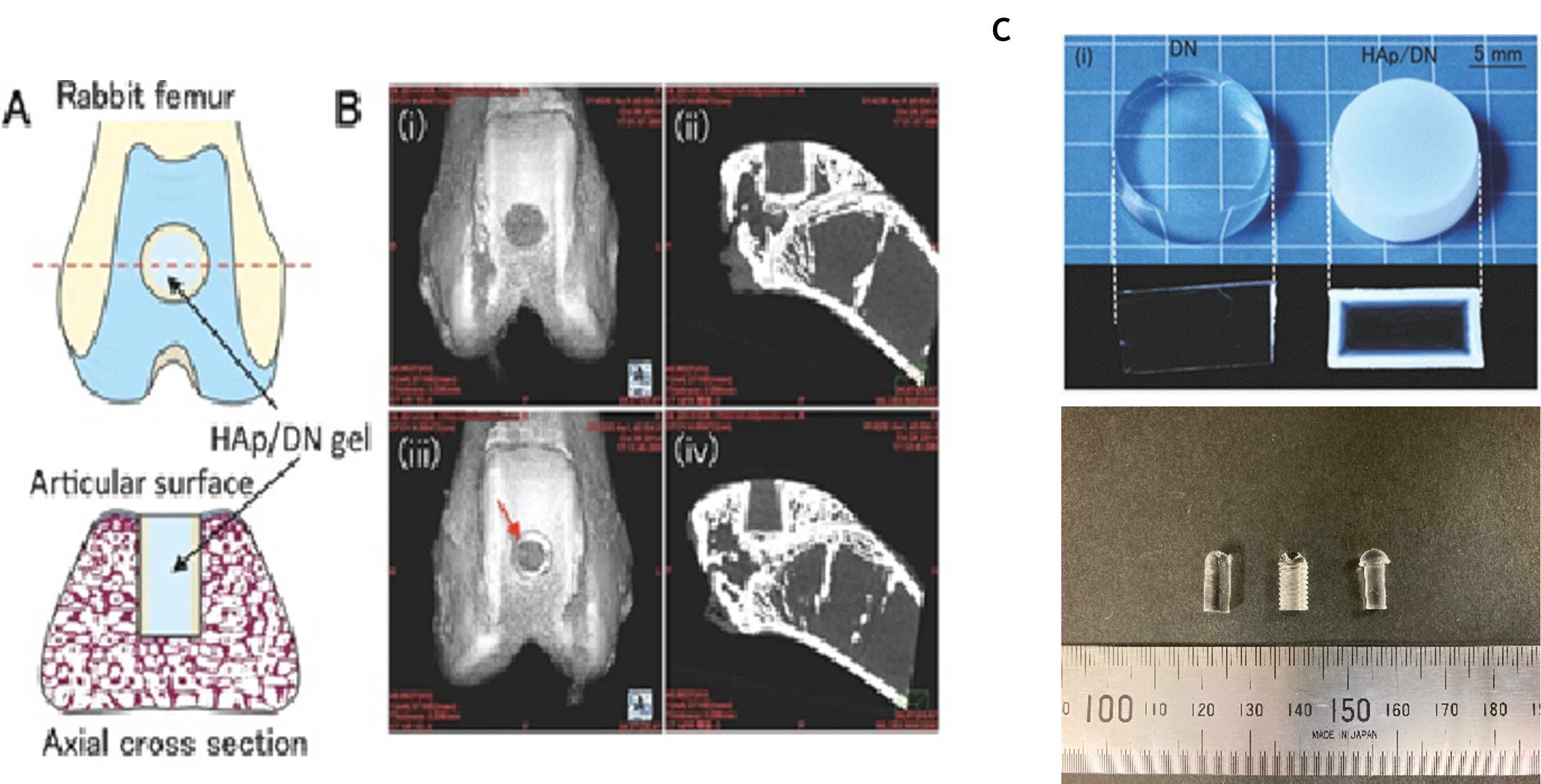 |
A : Schematic of HAp/DN gel plug implanted in rabbit femur
B : i,ii) μCT of DN or iii,iv) HAp/DN gels four weeks post-implantation
C : HAp-coated DN gel (upper right) and its implant material samples (lower figure, HAp uncoated)
Patent status
- Bone filler for cartilage tissue regeneration treatment
JP5166282,EP2143450,US9539366,CN101557839 - Hydrogel and hydrogel manufacturing method
JP7248967,US11492433,KR102422864,CN111727223 - Artificial cartilage implant and its manufacturing method
Pending (PCT level)
Researcher
Hokkaido University Faculty of Advanced Life Science Jian Ping Gong,Takayuki Kurokawa,Takayuki Nonoyama
Development Phase
This stage:
The stage where in vivo implant POC of HAp-coated DN gel has been completed
Next stage:
(1) Evaluation of mechanical strength, etc. by providing samples
(2) Establishment of manufacturing technology by providing DN Gel manufacturing equipment, molds
(3) Clinical development (evaluation of efficacy and safety through long-term animal studies, efficacy evaluation through clinical trials, procedure/device development, etc.)
We are looking for collaborative partners who are interested in development collaborations in (1), (2), and (3) above. We would be happy to start with a detailed explanation and discussion of the technology.
Project.ON-04230a


