Summary
- Hydrogen Boride “HB”, a novel material first synthesized at the University of Tsukuba, is attracting significant attention in the academic community for its unique properties. Numerous studies worldwide have reported its potential applications.
- Feature 1: Fuel Cell Electrode Material with Low Platinum Usage
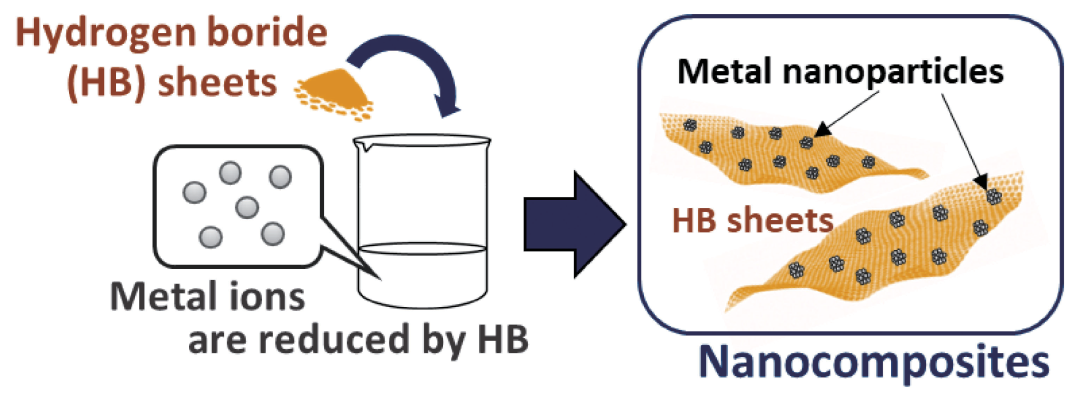
- HB acts as a reducing agent, leading to the formation of nanoparticles of precious metals (Ru, Pd, Pt, Ag, Au, etc.) on its surface.
- HB with PtPd alloy nanoparticles attached: an oxygen reduction reaction (ORR) catalyst that outperforms Pt/C catalysts in polymer electrolyte fuel cells (PEFCs). Using HB as the base material of the electrode can reduce the Pt consumption of fuel cells.
- HB with Ag alloy nanoparticles attached: enhances the activity of oxygen evolution reaction (OER) catalyst Co3O4. The HB+Ag is expected to be an auxiliary agent for hydrogen generation electrodes.
- High-entropy (multi-component) alloy nanoparticles such as Pt4FeCoNiCu attached material works as ORR catalysts that surpass the activity and durability of Pt/C catalysts in PEFCs.
- Feature 2: High-Density Hydrogen Storage Material
- High volumetric and mass hydrogen densities
- Hydrogen is released by heating, UV irradiation, and low electrical application
- Improved technology to release hydrogen by irradiation with visible light
- Feature 3: Efficient Carbon Monoxide Synthesis Catalyst
- HB reforms formic acid vapor to produce carbon monoxide and water, with high purity CO gas and minimal by-products.
- This is a new chemical material with the potential for further application development. We would like to discuss collaboration with manufacturers interested in the production of this material as a new catalyst technology.
Background & Technology
Hydrogen boride (HB) is a new material with a two-dimensional molecular structure, first synthesized by Professor Takahiro Kondo and member of his laboratory at the University of Tsukuba in 2017. It has broad and novel applications, including:
Fuel Cell and Water Electrolysis Electrodes Using Excellent Reducing Properties
HB reduces metals with higher redox potential than nickel, such as Sn, Cu, Pd, Ag, Pt, and Au. Surprisingly, the reduced metals appear as nanoparticles on the surface of the HB (see figure below) with particle sizes of 1-6 nm and high dispersion. Such special metal nanoparticles are expected to be even more active than metal particles with carbon or another base material.
The following applications have been reported HBs loaded with PtPd alloy particles have activity in the oxygen reduction reaction (ORR) (Paper 2). The activity is several times higher than the mass activity and specific activity of Pt/C typically used in polymer electrolyte fuel cells.
In another report (Paper 3), Pt particles on HB have a small size (2.5 nm), high density (up to 80 mass%), and high stability against sintering. The report also describes that a multicomponent metal compound (Pt4FeCoNiCu) made on HB exhibits significantly improved mass activity, specific activity, and very good durability over commercial Pt/C.
In another example (Paper 4), they report that Ag nanoparticles on HB combined with the oxygen evolution reaction (OER) catalyst Co3O4 have better oxygen evolution reaction (OER) activity than IrO2 catalyst.
Pt and metal alloy nanoparticles supported on HB are expected to enhance their intrinsic catalytic activity, leading to a reduction in the amount of precious metals used in electrodes of fuel cells and hydrogen generators. Replacement of traditionally used supports such as carbon black and graphene with HB is expected to lead to lower costs in fuel cells and water electrolysis.
 |
High-Density Hydrogen Storage Material
Hydrogen boride (HB) is expected to be utilized as a hydrogen storage material since it is literally a hydrogen-containing compound; the volumetric and mass hydrogen densities of HB are approximately 100 kgH2/m3 and 8.5 H mass%, respectively, which are comparable to other hydrogen storage materials.
HB has been shown to release hydrogen upon external stimulation such as heating above 150°C, irradiation with ultraviolet light, or application of electric current. Furthermore, in a recent joint research with Prof. Miyauchi of Tokyo Institute of Technology, we have developed a new compound of HB with a dye molecule attached to it. This improved HB releases hydrogen simply by irradiation with visible light (Paper 5).
Efficient Carbon Monoxide Synthesis Catalyst
The third special property of hydrogen boride is its ability to catalyze a dehydration reaction that reforms formic acid to produce carbon monoxide (see Presentation 1). This catalyst reforms formic acid vapor (120-300°C) to produce carbon monoxide and water. An outstanding feature is that the byproduct, carbon dioxide CO2, is not produced. The resulting carbon monoxide CO gas is very pure (100%), making it very easy to use in post-processes where CO is used, which we believe is a major industrial advantage.
Carbon monoxide is currently produced as a byproduct of gasification of coke or steam reforming of light hydrocarbons (natural gas, naphtha, etc.) to obtain hydrogen. However, the purity of CO is generally not high, making it difficult to handle and costly to refine in the subsequent stages of CO-based carboxylic acid synthesis, oxo reactions, and polyurethane synthesis. Formic acid reforming with hydrogen boride solves these problems and enables low-cost use of high-purity CO.
As described above, hydrogen boride, HB, is a cutting-edge material with very interesting properties, including aspects of catalysts and hydrogen storage materials. New applications and properties are still being discovered. The recent research status is detailed in a review paper by Prof. Kondo (Paper 6). We expect that there may be new properties other than those shown in this document. We would be happy to collaborate with your company to develop new technologies based on hydrogen boride.
Expectation
The University of Tsukuba is looking for a company that will conduct a manufacturer that produces hydrogen boride. It is expected to be effective in a wide range of fields, such as drastically reducing the amount of platinum used to fuel cells and hydrogen generators and improving the efficiency of reactions such as other oxygen reduction and generation reactions. We would like you to consider introducing this system as your company’s next-generation project.
We can provide you with information and samples as follows, and can discuss how we can assist you in considering and commercializing the introduction of this technology in your company.
- Response to your questions
- Meeting with you to explain the details of the technology
- Exchange of information under NDA
- Provide a small amount of samples under Material Transfer Agreement
- Joint research
- Patent licensing
Researcher
Prof. Takahiro Kondo, University of Tsukuba
Prof. Masahiro Miyauchi, Tokyo Institute of Technology
Patent & Publication
- Paper 1:Reporting on the synthesis of HB by Prof. Kondo
“Formation and Characterization of Hydrogen Boride Sheets Derived from MgB2 by Cation Exchange”
https://pubs.acs.org/doi/10.1021/jacs.7b06153 - Paper 2:Report on HB-supported PtPd nanoparticles for polymer electrolyte fuel cell electrodes
“Hydrogenated Boride-Assisted Gram-Scale Production of Platinum–Palladium Alloy Nanoparticles on Carbon Black for PEMFC Cathodes: A Study from a Practical Standpoint”
https://pubs.acs.org/doi/10.1021/acsami.2c08510 - Paper 3: Report on HB-supported Pt nanoparticles and High-entropy alloy nanoparticles
Hydrogenated borophene enabled synthesis of multielement intermetallic catalysts.
https://doi.org/10.1038/s41467-023-43294-z - Paper 4:Report HB-supported Ag nanoparticles promote the activity of oxygen evolution reaction (OER) catalysts.
“Ag nanoparticles modified crumpled borophene supported Co3O4 catalyst showing superior oxygen evolution reaction (OER) performance”
https://doi.org/10.1016/j.apcatb.2021.120529 - Paper 5: Report of the improved HB that releases hydrogen by irradiation with visible light
Visible-Light-Driven Hydrogen Release from Dye-Sensitized Hydrogen Boride Nanosheets
https://pubs.acs.org/doi/10.1021/acsami.4c07768 - Presentation 1:Report by Prof. Kondo et al. on the catalytic function of HB for formic acid reforming and carbon monoxide generation
“Investigation on the Catalytic Performance of Hydrogen Boride for Formic Acid”
https://doi.org/10.14886/jvss.2023.0_2Hp04 - Paper 6 :Review article by Prof. Kondo on HB
“Advancements in Freestanding Hydrogen Boride Sheets: Unraveling the Novel Properties of Borophane Polymorphs”
https://academic.oup.com/chemlett/article/52/7/611/7382019
Project.DA-04473b


