Advantages
- Facilitating the culture of suspension-type human primary hepatocytes in various formats, including 2D, sandwich culture systems, 3D spheroid, passaging, and cryopreservation, all at affordable prices.
- This leads to high-quality reproducible, polygonal hepatocytes that express multiple essential hepatic markers, including maturation markers, drug-metabolizing enzyme, uptake, and efflux transporters that greatly expand its applications
- Long-term culture up to 1 month is possible, which is expected to be applied to research requiring long-term culture, such as chronic toxicity studies.
Technology Overview & Background
Primary human hepatocytes are a commonly used cell product in drug evaluation studies and can be classified into two types: Plateable cells, which adhere to a plate, and Suspension cells, which cannot adhere to a plate. Both are obtained from living human livers, but it is not known which is the outcome until the cells are seeded and their adhesion status is checked, and the underlying reasons for this variability remain unclear.
Plateable cells are versatile because they can be cultured for several days and can be used for several applications such as cell-cell interactions, transporter function, induction of drug-metabolizing enzymes, and evaluation of cytotoxicity drugs. On the other hand, Suspension hepatocytes are floating, lose attachment to the plate surfaces, and have a short lifespan of only a few hours. These limitations significantly reduce their utility. Plateable cells are more expensive. So, if suspension-type hepatocytes can be converted to plateable-type, the application of economically lower-priced human primary hepatocytes could be expanded.
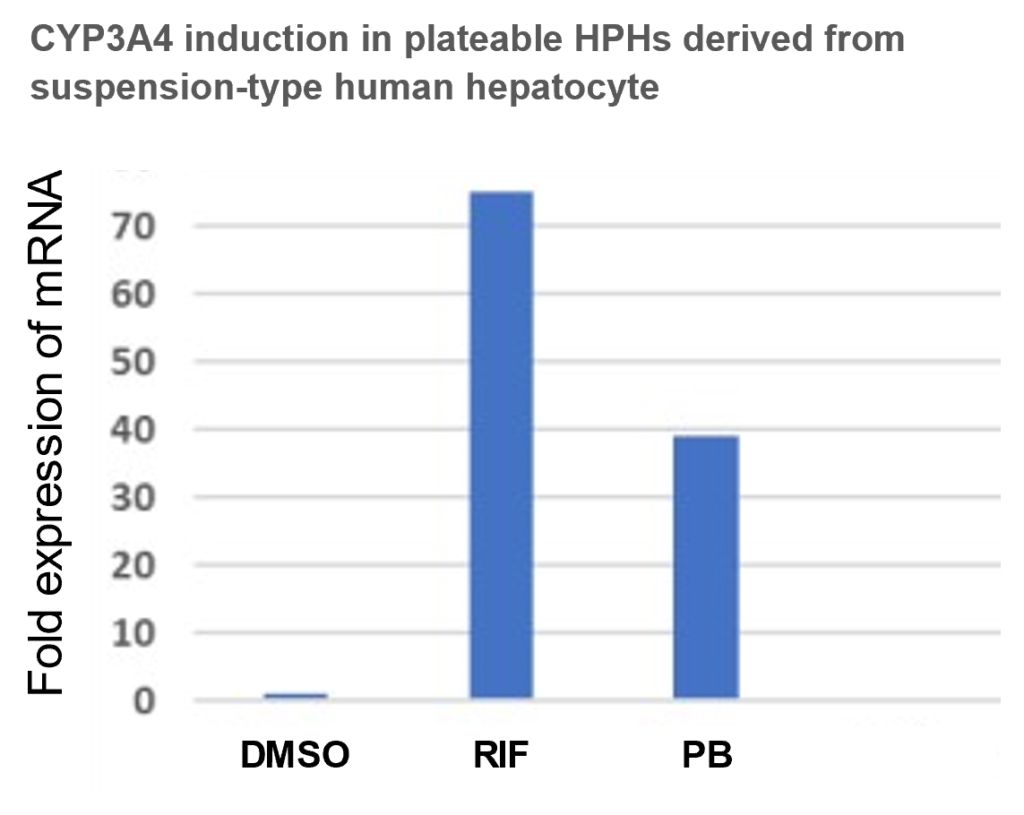
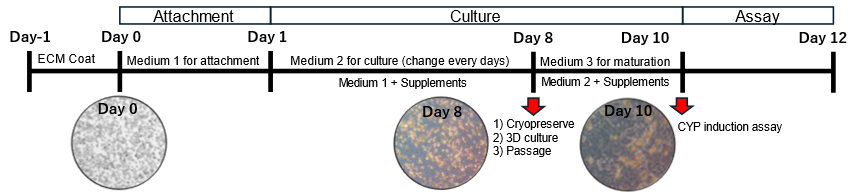
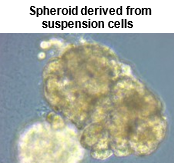
Based on their previous knowledge of stem cell culture and differentiation induction, the inventors found that Suspension-type hepatocyte can be cultured on ECM-coated plates and become cells that can adhere to the plates by adding supplements such as small molecules. It was confirmed that the Plateable cells induced using the present invention can be used for pharmacokinetics experiments and can be cultured as a 2D, 3D spheroid, passaging, and cryopreserved. Furthermore, the cells can be cultured for a long period, up to one month, and are expected to be used in pharmacokinetics studies and in research on chronic toxicity tests such as drug-induced liver injury, which require long-term culture.
Data
 |
 |
Patent(s)
Pending in Japan (unpublished)
Principal Investigator
Prof. Tamihide Matsunaga(Nagoya City University)
Expectations
We are looking for a company that develops cell products for research that would consider commercialization and practical application of primary hepatocyte products through the licensing introduction of this invention. We are available for direct meetings with researchers and disclosure of unpublished data and other information by concluding a confidentiality agreement with Nagoya City University.
Project.WL-04710