Advantage and Core Benefit
- Strong anti-tumor effects are expected from the combination of IDO1 inhibitors and COX-2 inhibitors
- Combination with NSAIDs is expected to have applications in veterinary medicine
- An original evaluation system using IDO1 stable-expressing cell lines allows selection of combination drugs in a short time, efficiently and with a high degree of accuracy
Background and Technology
The immune escape mechanism of cancer cells is one of the key targets for the development of anticancer drugs. Kynurenine, a metabolite of tryptophan (Trp), is a regulator that induces immune tolerance to cancer, and indoleamine-2,3-dioxygenase 1 (IDO1), an enzyme that modulates this pathway, is a new target for cancer immunotherapy. Several IDO1 inhibitors have been developed as cancer drug candidates and have progressed to clinical trials, but have not shown the expected advantages, leading to many drugs being discontinued. This suggests that IDO1 inhibition alone is insufficient for therapeutic efficacy, and new development strategies, such as combinations with other drugs, are needed.
The inventors established a mouse transplantation model using an IDO1 high-expressing strain (CT26-IDO1) and performed transcriptome analysis of the tumor. The results showed that COX2 expression was significantly up-regulated in the CT26-IDO1 transplantation model compared to the wild-type CT26 transplantation model.
Based on these results, an IDO1 inhibitor (Epacadostat) and a COX-2 inhibitor (Celecoxib) were administered in combination to evaluate their ability to inhibit CT26-IDO1 tumor growth. As a result, significant tumor growth inhibition was observed in the combination-treated group. Furthermore, the therapeutic effect of the combination of an IDO1 inhibitor and a COX-2 inhibitor (Piroxicam) on canine urothelial carcinoma cases with high expression of IDO was also confirmed, indicating that the combination is promising for development as an animal drug.
Data
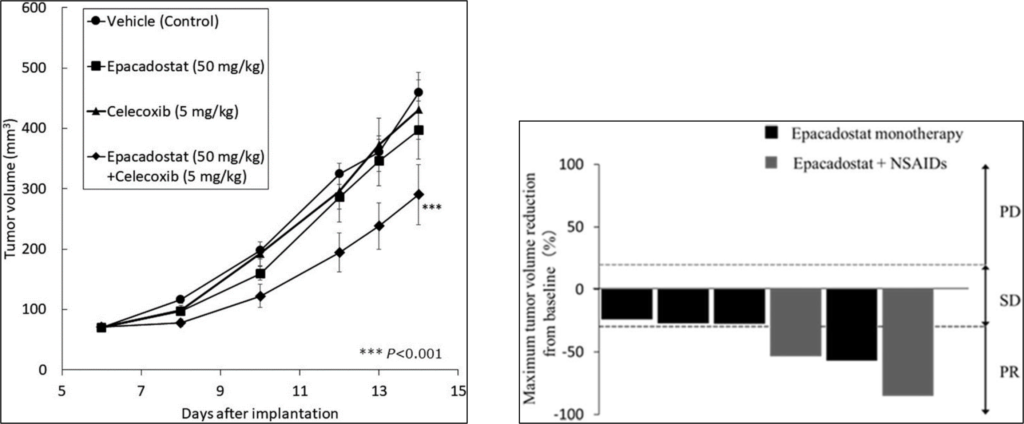 |
- Epacadostat (50 mg/kg) and celecoxib (5 mg/kg) in a CT26-IDO1 transplant mouse model showed no effect as single agents, but in combination.
- Epacadostat alone (5 mg/kg, orally twice daily; ■ in the figure) produced partial response (PR) in 25% (1 of 4 patients) and stable (SD) in 75% (3 of 4 patients) of canine urothelial carcinoma cases Epacadostat and Piroxicam (0.3 mg /kg, orally once daily: ■ in the figure), partial response (PR) was 100% (2 of 2 patients). No serious adverse reactions of Grade 3 or above were observed.
Patent & Publication
Pending in Japan (Unpublished)
Researcher
Dr Akira Asai (University of Shizuoka) and five other project members
Expectations
Patent Licenses are offered to companies interested in the clinical development of combination therapies with COX2 inhibitors as a strategy for advancing the development of IDO1 inhibitors. We also welcome collaborations with companies interested in developing animal medicines into cancer therapeutics.
Project.WL-04992


