Advantages
- Low side effect risks due to the supplementation of intestinal metabolite/bacteria.
- New prevention/treatment concept and used in combination with existing drugs.
Technology Overview & Background
Ulcerative colitis (UC) is a chronic inflammation from the colon to the rectum. Although several classes of medication have been developed, only about 50% of patients respond to them, and many patients still suffer from symptoms with repeated remissions and exacerbations.
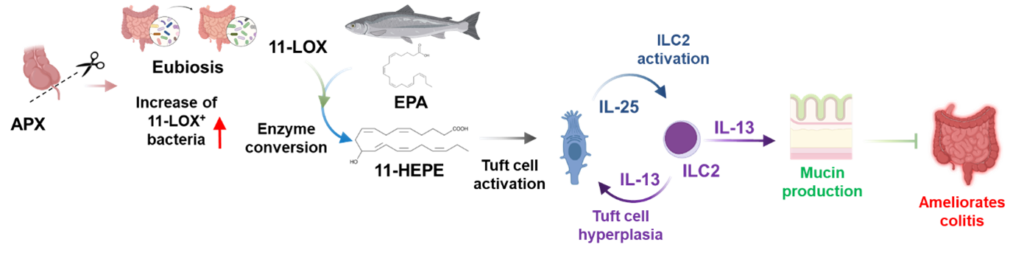
The research group of Prof. Kazuyo Moro, known for the discoverer of type 2 innate lymphocytes (ILC2), has found that changes in intestinal flora caused by appendectomy alleviate the symptoms of UC with improvement of intestinal epithelial barrier function by enhanced mucin production through tuft cell > ILC2 > goblet cell activation cascade.
They identified 11-hydroxy-eicosapentaenoic acid (11-HEPE), a metabolite of eicosapentaenoic acid (EPA), as the molecule contributing to tuft cell activation/augmentation. Bacteria that enzymatically convert EPA to 11-HEPE via 11-LOX enzyme were also identified. 11-HEPE and 11-LOX expressing bacteria are expected to be applicable for the prevention, remission maintenance, and potentially treatment of UC, as well as other symptoms of inflammatory bowel disease (IBD), Crohn’s disease and unclassified IBD.
 |
Data
- In a dextran sulfate sodium (DSS) induced UC mouse model, enteral administration of 11-HEPE 7, 5, 3 and 1 day before the start of DSS drinking alleviated weight loss and colon shortening on day 8.
- Oral(intragastric) administration confirmed dose dependent efficacy of 11-HEPE with DSS model.
- Identified intestinal bacteria that metabolize EPA to 11-HEPE.
- An observed increase in the number of tuft cells in intestinal epithelial tissue in humans who have undergone an appendectomy and patients in UC remission. Intestinal tuft cells reportedly decrease in UC patients.
- 11-HEPE induced tuft cell differentiation in human colonic organoid.
Patent(s)
Patent pending (WO/2025/023183)
Researchers & Academic Institution
Professor Kazuyo Moro, PhD (The University of Osaka Graduate School of Medicine, Japan)
Expectations
- We are seeking pharmaceutical/biotech companies interested in developing 11-HEPE or its derivatives as therapeutic agent, and/or 11-HEPE producing bacteria for FMT(fecal microbiota transplantation).
- Considering commercialization as supplement is welcome.
- Collaborative research is also welcome.
- We can also arrange a non-confidential meeting with the inventors.
Project.KJ-04988


