MASH Fibrosis Biomarkers
Diagnosis of liver fibrosis in MASH using serum IgA protein bound A2Fbisect glycans and their precursor glycans as biomarkers
Advantages
- High specificity. Does not react with fibrosis caused by other types of hepatitis.
- Can be detected using blood samples and simple kits.
Background and Technology
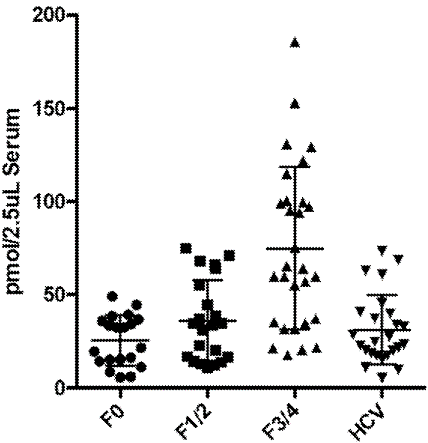
| The progression of liver fibrosis in metabolic dysfunction-associated steatohepatitis (MASH, formerly known as NASH) leads to the development of liver cirrhosis and liver cancer, so early detection of fibrosis is an important issue. The most accurate evaluation of liver fibrosis is histological evaluation by liver biopsy, but it places a heavy burden on patients and carries the risk of infection. For this reason, blood hyaluronic acid, type IV collagen, M2BPGi, FIB-4 index, etc. are used as non-invasive evaluation. However, these are also increased in other diseases and inflammation, so diagnostic markers with high specificity for MASH are required.
We found that the expression of A2F bisect glycans in the blood increases with the progression of liver fibrosis in MASH. Furthermore, we demonstrated that A2F bisect glycans and their precursors bound to the carrier protein IgA could be biomarkers for liver fibrosis in MASH. |
 |
Patents
- Patent pending in Japan, the United States, and China (Publication number: WO2021/010349)
Principal Investigator
Naoya Sakamoto (Graduate School of Biomedical Science and Engineering Faculty of Medicine, Hokkaido University)
Current Stage and Next Step
- To date, we have developed a prototype kit to detect A2F bisect glycans and their precursor glycans bound to IgA protein in the blood and have evaluated over 100 blood samples from MASH patients. As a result, we have confirmed high diagnostic performance.
- A research project supported by AMED (Japan Agency for Medical Research and Development) is underway. We will continue to improve the measurement kit, increase its accuracy, and clarify its mechanism.
- We are currently seeking partner companies to develop a measurement kit and develop in vitro diagnostics with the aim of having the diagnostic drug covered by insurance.
Project.BK-04714



