Advantages
- Novel target and first-in-class medication.
- Repositioning/reformulation strategies applicable.
- Potential indications for several rare diseases with cilial disfunction.
Background and Technology
Malfunctions in the development and function of cilia cause diseases called ”ciliopathies.” As pathogenic mechanism is unclear and no treatment has been established, some ciliopathies are designated as intractable diseases (e.g. polycystic kidney disease, retinitis pigmentosa (RP), diseases related to Joubert syndrome, nephronophthisis, macular dystrophy). In Japan, about 24,000 patients suffer from retinitis pigmentosa. A major proportion of RPs is known to be caused by cilial dysfunction.
We discovered that intestinal cell kinase (ICK) and Male gene associated kinase (MAK), one of the causative genes for RP, complimentary control the function of intraflagellar transport (IFT), which is essential for cilial formation and function.
This technology is a use of fibroblast growth factor receptor (FGFR) inhibitors, which activate ICK, for the treatment of ciliopathy by stabilizing IFT function of patients with not only MAK but other IFT related gene mutations. FGFR inhibitors are being developed primarily for cancer therapy, and some of them have been commercialized. Repositioning could reduce the development risk.
Data
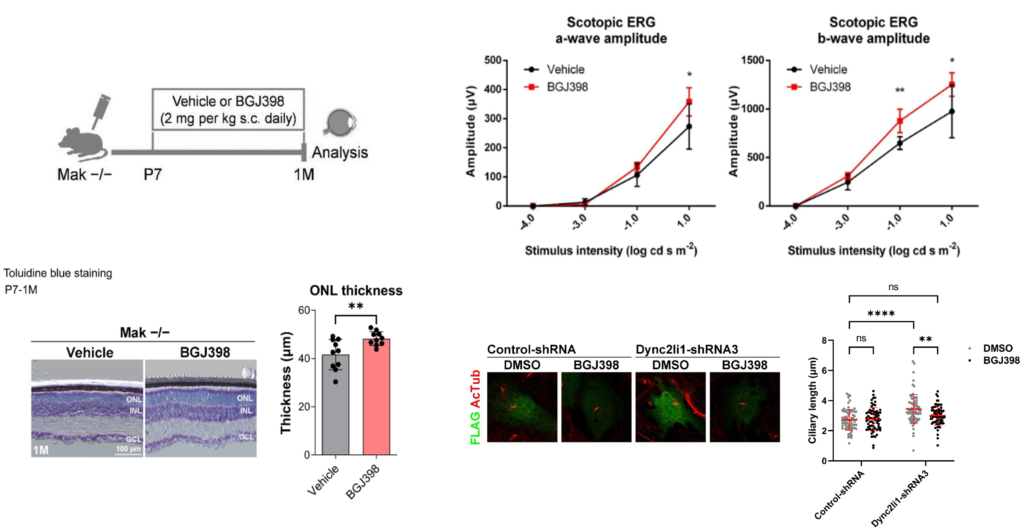
- In Mak-deficient mice (RP model), photoreceptor degeneration and light response dysfunction in retina were alleviated by administration of FGFR inhibitors BGJ398 and AZD4547 (fig).
- Ciliary abnormalities in cells caused by dynein function inhibition (knockdown of Dync2li1, another ciliopathy causing gene) were suppressed by treatment of BGJ398/AZD4547.
 |
Patent/Publications
Journal: Life Science Alliance Sep 2024, 7 (11) e202402880;
DOI: 10.26508/lsa.202402880
Patent: WO2025/178019
Researcher
Prof. Takahisa Furukawa, Institute for Protein Research, Osaka Univ., Japan
Expectations
- The University of Osaka is seeking companies who could license and commercialize this technology by licensing and/or collaborative R&D.
- Pharmaceutical companies who have focuses on ophthalmology, rare diseases, repositioning would be particularly welcome.
- Details can be disclosed under CDA.
Project.KJ-04809


