Advantages
- Rapid preparation in 3-10 days, amplified into 10^5-10^7 cells.
- Relatively minimally invasive tissue like buccal pad-fat, gum, as well as dental pulp can be applied.
- More effective than DPSC transplantation, expected neural regeneration as well as neuroprotection.
- Conventional stereotactic neurosurgery techniques applicable for local administration.
Technology Overview & Background
In ischemic stroke and spinal cord injury, cell transplantation within 2 weeks of onset is expected to restore function by regenerating the neural network. However, generally it takes 6 weeks to obtain autologous cells for transplantation, which is not compatible with treatment in the acute and subacute phases, and off-the shelf allogeneic cells are difficult to be viable due to rejection.
This technology is based on the finding that small oval cells obtained from the culture, not adherent to substrate, by direct neural differentiation culturing of oral mesenchymal tissue cells for 48 hours are neural stem cells, and further 24 hour-culturing forms spheroids consisting of nervous system cells; neural stem cells, astrocytes, oligodendrocytes, and immature and mature neurons. The cell transplantation with stereotactic neurosurgery is expected to restore the neural networks, which is difficult with other stem cell therapies.
Data
- In human dental-pulp-derived induced neural cells (DP-iNC), Nestin (immature neural stem cells), Beta III tubulin (neurons), NF200 (large medullary neurons), MBP (glial cells), GFAP (Astrocytes) were detected. Normal diploid cells were >95% (commonly <80%).
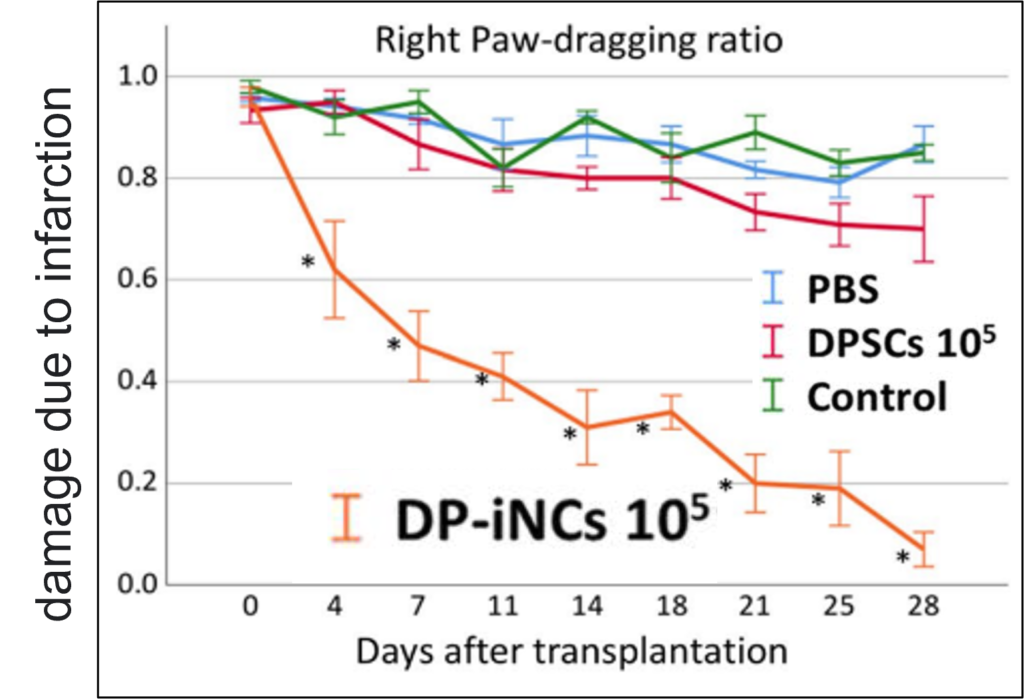
- DP-iNC and dental pulp stem cells (DPSC) were transplanted into the peripheral area of infarcted region 5 days after stroke in left middle cerebral artery permanent occlusion of immunodeficient mice. Neural circuit formation by transplanted cells, and significant improvement in symptoms (Fig) were observed.
- Neural differentiations were confirmed by buccal pad-fat and gum.
 |
Publication(s)
Stem Cell Reviews and Reports (2022) 18:595–608, DOI: https://doi.org/10.1007/s12015-021-10223-w
Patent(s)
PCT/JP2020/019290, pending in JP, US, GB, DE.
Researchers & Academic Institution
Aiki Marushima, Ph.D. (Associate Professor, Tsukuba University, Japan), et al.
Development Stage & Future Research Plans
The researchers aim to conduct POC in large animals, and investigator-led clinical research under funding.
Expectations
We are looking for pharmaceutical companies to develop this technology further under the license.
We can disclose unpublished data under CDA with University of Tsukuba or arrange a non-confidential meeting with the researcher.
Project.KJ-04954


