Advantage and Core Benefit
- Avoids bladder infusion, which is highly burdensome and uncomfortable for patients
- Expected to be more effective than the recently approved DMSO infusion therapy
- Can be developed by expanding the application of integrin inhibitors under development for other disease
Background and Technology
Interstitial cystitis (IC) is a chronic inflammatory disease characterized by symptoms such as frequent urination and bladder pain. It is estimated that there are approximately 1.3 million patients in the United States and 250,000 patients in Japan. Multiple factors are thought to be involved in the pathogenesis of IC, including dysfunction of the bladder mucosa and immunological mechanisms, but these have yet to be elucidated. Bladder hydrodistension is a treatment for IC, but the response rate is 50% and the duration of response is less than 6 months, which is not always effective, and relapse of symptoms is also a problem. Recently, bladder injection therapy with DMSO has been approved, but there is no curative therapy, and the disease has a high unmet need.
The inventors performed a comprehensive genetic analysis of bladder tissue from a combination of human interstitial cystitis patients and model animals and found that a pathway related to integrins with RGD binding sites was activated. Based on these results, the inventors administered RGDS peptide, a known inhibitor, to the IC animal model and found that it improved symptoms of urinary frequency. An effect on pain has also been suggested (data not disclosed). Similarly, Cilengitide (an integrin αvβ3, αvβ5 inhibitor), whose development was discontinued in Phase 3 due to inadequate efficacy in clinical development for cancer, also improved symptoms of urinary frequency.
Data
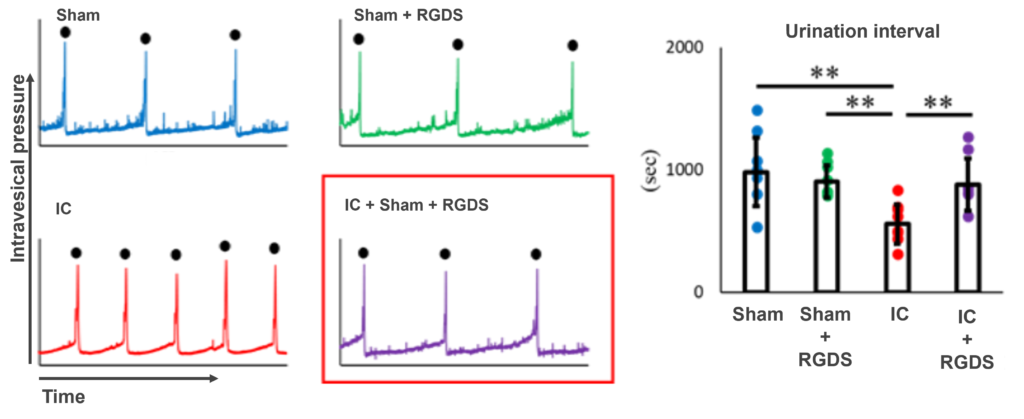
- Intraperitoneal administration of RGDS peptide to a bladder-injected IC model with hydrochloric acid improved the urinary voiding interval to the same level as the Sham group. Intraperitoneal administration of Cilengitide in the same model showed the same effect.
- The effects of RGDS peptide and Cilengitide on the voiding interval were greater than those of DMSO
 |
Patent & Publication
PCT/JP2022/033623
Researcher
Dr. Yuji Hotta (Nagoya City University, Graduate School of Medicine, Clinical Pharmacy) and Dr. Seiji Matsumoto (Asahikawa Medical University, Research Promotion Division)
Expectations
We would like to partner with a company that seeks to commercialize drug discovery based on this research. We believe there is potential to expand the application of Cilengitide and to apply integrin inhibitors that are being developed for other diseases to IC. We would like to collaborate with companies that specialize in the development of repositioning agents, companies developing drugs that target integrins, and companies interested in developing drugs to treat urologic diseases.
Project.WL-03852a


