Advantage and Core Benefit
- Highly accurate prediction of efficacy of first-line drugs for liver cancer drug therapy, which have many options but response rates of about 30% in each case
- Immune status of patients with liver cancer can be evaluated by next-generation sequencers.
Background and Technology
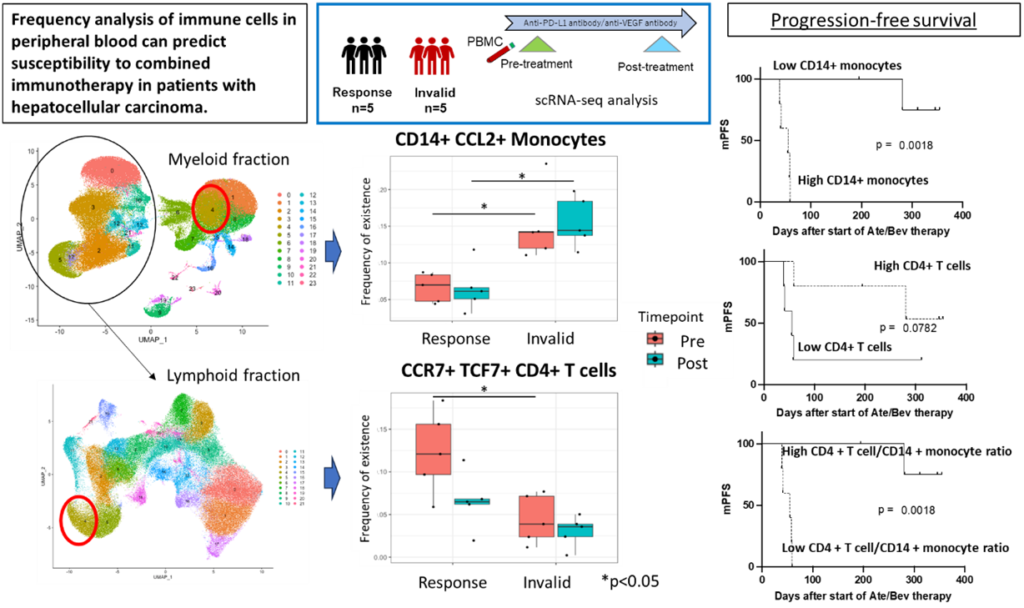
Several immune checkpoint inhibitors are now available for drug therapy of advanced hepatocellular carcinoma. Among them, atezolizumab/bevacizumab therapy (Atezo/Bev), a combination of anti-PD-L1 and anti-VEGF antibodies, is the first-line therapy. It has been reported that the presence of CD8-positive T cells in the tumor microenvironment influences therapeutic efficacy, but the relationship between systemic immune dynamics and response to Atezo/Bev therapy is unclear, making it difficult to predict efficacy in advance.
The inventors profiled the immune dynamics of advanced hepatocellular carcinoma patients by scRNA-seq analysis, selecting 5 response cases and 5 initial failure cases among the patients who were injected with Atezo/Bev therapy. The results showed that the response cases had a higher frequency of CCR7-positive and TCF7-positive naive memory T cell subsets among pre-treatment CD4-positive T cells and a lower frequency of CCL2-positive and CXCL2-positive subsets among CD14-positive monocytes. This suggests that analysis of immune dynamics in PBMCs before treatment may be able to predict the therapeutic effect of combined immunotherapy in patients with advanced hepatocellular carcinoma.
Data
- Sequences in 2*10^6 PBMCs were classified into 16 subtypes based on each genetic marker, and the response cases had a higher frequency of naive memory CD4 T cells before treatment and a significantly lower frequency of CCL2-positive and CXCL2-positive CD14+ monocytes.
 |
Patent
Pending (Unpublished)
Researcher
Dr. Takahiro Kodama (The University of Osaka)
Expectations
We are looking for diagnostics and biotech companies that are interested in licensing this invention to develop diagnostic technologies for drug response rates for liver cancer patients. We are particularly interested in collaborating with companies interested in developing diagnostic technologies using next-generation sequencers and analyzing PBMCs.
Project.WL-04754


