Advantage and Core Benefit
- Direct access to bacteria via membrane vesicles may enable treatment against specific bacteria
- It is expected to inhibit the unintended transformation of common bacteria into resistant strains
- Enables mass production of membrane vesicles through cell lysis (Explosive cell lysis)
- Increased potential for delivery to bacteria even in the presence of barriers such as biofilms
Background and Technology
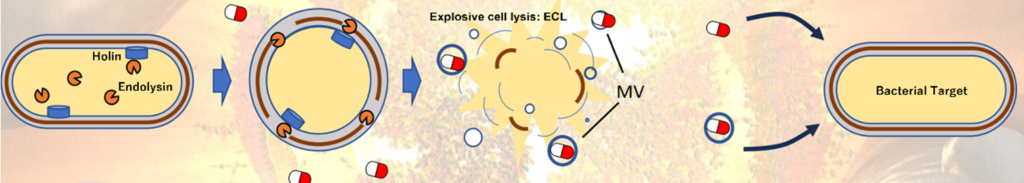
It is known that bacteria release membrane vesicles (MVs) outside the cell that are involved in exchange of membrane proteins between bacteria, horizontal gene transfer, and communication via signaling molecules. In fact, membrane vesicles have been put to practical use as carriers for vaccines and genome editing to introduce nucleic acids into cells. However, the mechanism by which membrane vesicles are produced is not understood, and the “quality” of the membrane vesicles cannot be controlled. Moreover, the yield is not high.
The inventors have established a method for producing membrane vesicles that efficiently incorporate the target substance by utilizing the mechanism by which Gram-negative bacteria form membrane vesicles. When the expression level of endolysin, a cell wall degrading enzyme, exceeds a certain level in Gram-negative bacterial cells, the cells undergo explosive cell lysis (ECL), causing cell death, and the cell membrane fragments released by ECL reassemble to form membrane vesicles. Furthermore, they found that by including target substances in the culture medium when ECL is induced, the target substances are efficiently internalized into the membrane vesicles (see figure below). This makes it possible to create membrane vesicles encapsulating antibiotics, nucleic acid drugs, and components for gene editing, and is expected to lead to the development of drug and gene editing therapies against specific bacteria.
 |
Data
|
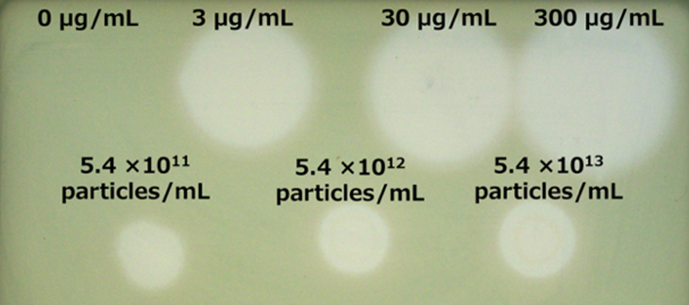 |
Patent & Publication
Patent Pending: JP2022-77676
Researcher
Dr. Masanori Toyofuku (University of Tsukuba)
Expectations
We are interested in collaborating with companies (pharmaceutical and biotech companies) that are interested in using this technology to develop highly active antibiotics and gene editing technology against highly specific bacteria. We envision a collaboration in which MVs are created using this technology for payloads such as antibacterial agents or nucleic acid drugs that the companies have, and the effects are verified. We are also interested in collaborating with a company that provides drug discovery support services and is developing membrane vesicle technology such as exosomes to establish it as a fundamental drug discovery technology.
Project.WL-04797a


