Advantages
- Versatile peptide intermediate, solubilization unit targeting C-terminal carboxyl groups
- Solubility can be added at the late-stage, enabling handling with less trial and error
- Available for peptide synthesis using unnatural amino acids
- Aspartimide-free synthesis of aspartic acid-containing peptides
Background & Technology
Chemical synthesis of proteins is generally performed using the native chemical ligation (NCL) method, in which peptide thioesters chemoselectively react with N-terminal cysteine-containing peptides in aqueous solvents, but the low solubility of the synthetic intermediate makes analysis and purification difficult. The temporary attachment of hydrophilic tags onto poorly soluble peptides improves their aqueous solubility. Despite its advantage, the method has a potential limitation because the tag moiety must be introduced during solid-phase peptide synthesis (SPPS), and thus the peptide segments with the solubilizing tag must be resynthesized if the solubility-enhancing property is insufficient. In this context, the late-stage solubilization of poorly soluble peptides, which enables a choice of types of solubilizing tags even after the synthesis of peptides of interest, should be useful.
We have developed a method for the site-selective and late-stage introduction of solubilizing tag molecules to unprotected peptide through chemoselective orthogonal reaction of hydrazide with aldehyde. The hydrazides reacted with the solubilizing tag can be converted to carboxylic acids by copper(II) ion treatment. This method has been extended to other peptide segments than the C-terminal segments by modifying the linker to afford hydrazide upon tag removal, and the applicability of this method has already been demonstrated.
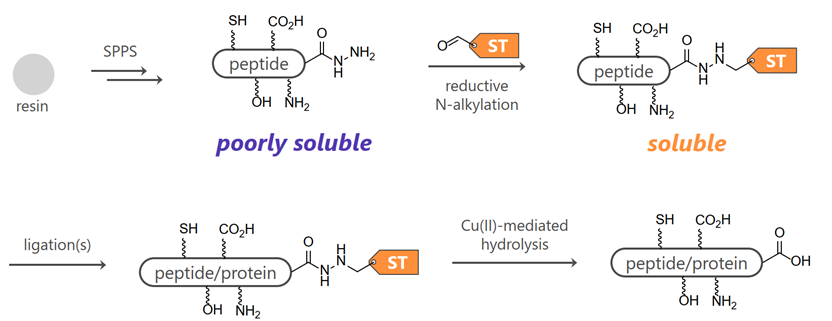 |
In the solid-phase synthesis of aspartic acid (Asp)-containing peptides by the Fmoc method, the key is how to suppress the formation of aspartimide byproducts. In the past, introduction of a protecting group on the nitrogen atom of the amide bond in the backbone or protection of the side chain carboxyl group with a bulky ester have been proposed as methods to suppress the formation of aspartimide; however, there is no de facto standard in suppression of aspartimide formation. In this laboratory, we have developed a versatile method to inhibit aspartimide formation by introducing hydrazides, which are more stable carboxylic acid derivatives than esters, into the Asp side chain. This method is also applicable to peptide synthesis under microwave irradiation conditions.
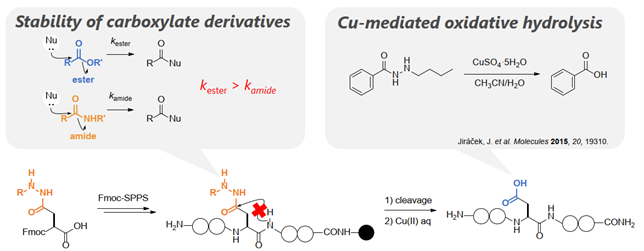 |
Researcher
Shizuoka University, Department of Applied Chemistry and Biochemical Engineering
Assistant Professor Kohei Sato
Publication
Sato, K. et al. Org. Lett. 2021, 23, 1653.
Sato, K. et al. Chem. Pharm. Bull. 70, 707–715 (2022)
Expectation
The laboratory has extensive knowledge of protein chemical synthesis methods, hydrazide-based synthetic tools and know-how, and semi-synthesis of thioamide-containing proteins. We are looking for technical consulting or joint research/development with partner companies who wish for the following
(1) Want to consider distributing our laboratory’s tools as a synthesis unit
(2) Need knowledge to solve, develop, or optimize your own synthesis process
(3) Interested in industrialization of this process through development of mass production technology
We would be happy to start with a detailed explanation and discussion of the technology.
Project No.ON-04891


