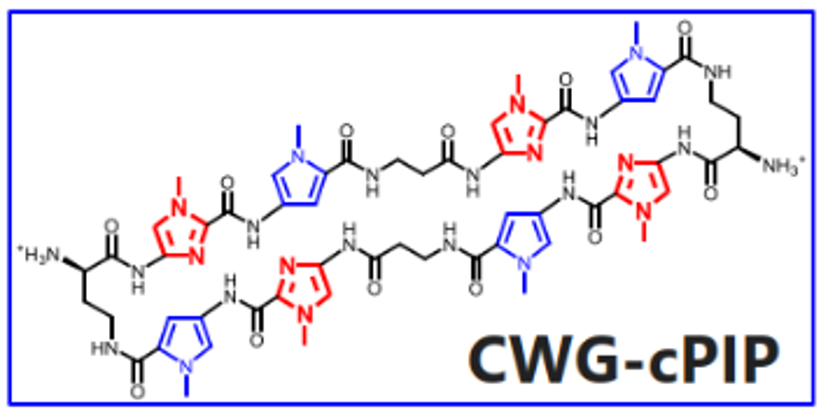
Advantages
- Transferred into the brain by peripheral intravenous administration, High cell permeability, nuclear translocation, and low cytotoxicity; no need for DDS
- Suppresses transcription selectively to abnormally extended repeats, no off-target effects.
- Effective against abnormally expanded repeats in both translated and untranslated regions.
Background and Technology
Abnormal expansion of CAG/CTG (CWG) sequence repeats in genomic DNA causes serious genetic neurological diseases, triplet repeat diseases. e.g. CAG repeat expansion in the translated region: Huntington’s disease/HD, CTG repeat expansion in the UTR: myotonic dystrophy type 1/DM1.
 |
Reference
Principal Investigator
Norifumi SHIODA, Prof. (Institute of Molecular Embryology and Genetics, Kumamoto University)
Current Stage and Next Step
- Confirmed a decrease in RNA aggregates/abnormal proteins in the DM1/HD mouse neuron model and patient-derived fibroblasts (in vitro).
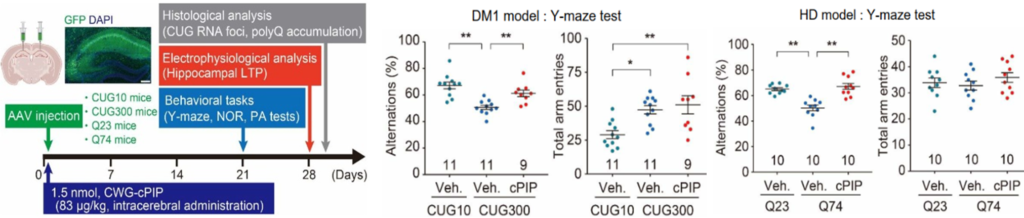
- In DM1/HD brain-specific CWG repeat-expressing mice, a decrease in RNA aggregate-positive cells/polyQ aggregate-positive cells, electrophysiological improvement, and cognitive function improvement (upper figure) by i.c.v. administration of cPIP were confirmed (in vivo).
- Confirmed that cPIP moves into the brain after peripheral intravenous administration, and long-term efficacy evaluation is currently underway.
- We are looking for collaborative partner companies for preclinical and clinical development.
Project No. BK-04516