Advantages
- Unique artificially modified nucleic acid ASO accumulates in the kidneys when administered subcutaneously to animal models.
- Improvement of renal function through multiple mechanisms of action (suppression of cyst formation, suppression of renal fibrosis, improvement of vascular endothelial disorder).
Background and Technology
ADPKD is a type of cystic kidney disease and is a congenital disease caused by a single gene PKD1/2 mutation with autosomal dominant inheritance. The incidence is 1 in 3,000 people, and it is said to be the most common single gene mutation disease. Cysts form in the liver and kidneys, resulting in kidney enlargement, and approximately half of patients in their late 60s require maintenance dialysis. The world’s only vasopressin V2 receptor antagonist has been approved as a specific treatment for slowing the progression of the disease, but its effectiveness is still not sufficient.
We identified a therapeutic target molecule for ADPKD, developed a stabilized ASO using a unique artificially modified nucleic acid to knockdown the molecule, and confirmed its effectiveness.
 |
 |
Reference
- Patent pending (Unpublished yet)
Principal Investigator
Noritoshi KATO (Department of Nephrology, Nagoya University Hospital)
Current Stage and Next Step
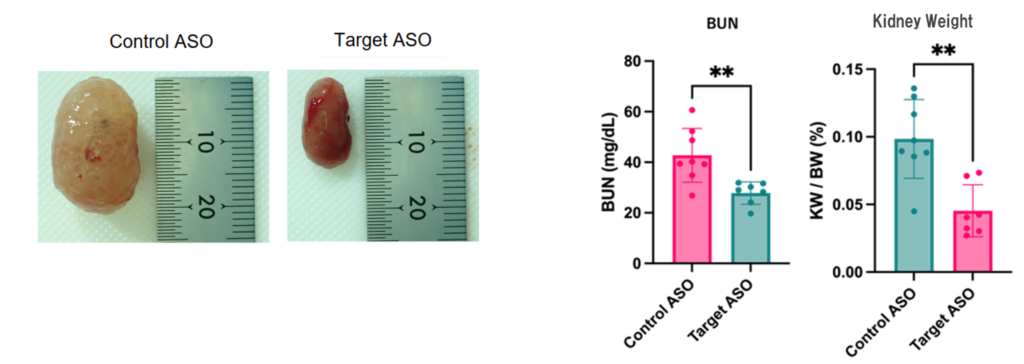
- Our original artificially modified nucleic acid ASO accumulated in the kidney when administered subcutaneously to cystic kidney model animals and showed a stable knockdown effect in vivo. The therapeutic effects showed inhibition of cyst formation, inhibition of renal fibrosis, improvement of markers related to improvement of vascular endothelial damage, inhibition of renal hypertrophy, and improvement of renal function. In addition, the unique artificially modified nucleic acid showed a more stable effect on suppressing renal hypertrophy than LNA, a well-known artificially modified nucleic acid.
- Currently analyzing the mechanism of action in vitro.
- We are looking for collaborative partner companies for non-clinical and clinical development of ASO therapeutics for ADPKD.
Project No. BK-04726


