Advantages
- A single dose is expected to produce long-term effects (more than one year).
- Maintains the physiological function of α-Syn.
- Effective for both sporadic and hereditary cases.
Current Stage and Key Data
Preclinical research stage
- Identification of multiple SSOs that efficiently induce SNCA splice switching (promotion of low-aggregation variants).
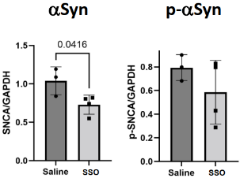
- Intracerebroventricular administration (once immediately after birth) of the SSOs to Parkinson’s disease model mice (human SNCA E46K BAC Tg +/-) induced sustained splice switching and reduction of aggregating α-Syn protein in the cerebral cortex and brainstem (13 months after administration).
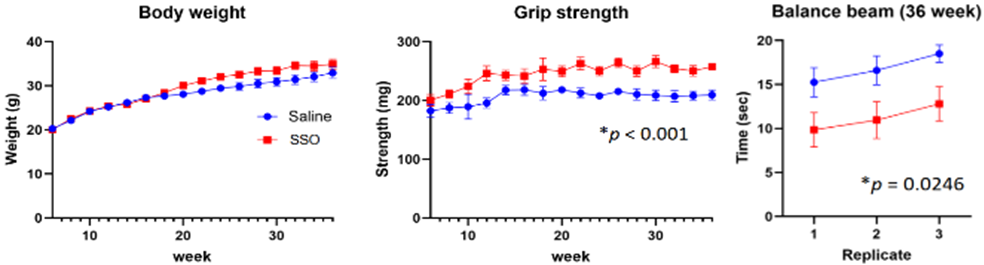
- Administration of the SSO reduced motor dysfunction measured by grip strength, balance beam test, and open field test in Parkinson’s disease model mice (+/+) (36 weeks old).
- Histological evaluations showed improvement in degeneration of dopaminergic neurons in the striatum (putamen) (13 months after administration).
 |
 |
Partnering Model
Seeking an exclusive license partner company
- Examples of potential partners: Biotech/pharmaceutical companies focusing on neurodegenerative diseases such as Parkinson’s disease and/or nucleic acid medicines.
Background
α-Synucleinopathies, such as Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy, are neurodegenerative diseases characterized by the abnormal accumulation of α-Syn aggregates. Research on antisense oligonucleotides (ASOs) targeting SNCA knockdown has been reported. However, a major limitation is the need for repeated administration (e.g., via lumbar puncture) due to the insufficient duration of efficacy. In addition, α-Syn is thought to be involved in regulating synaptic function, raising concern that its knockdown may inhibit neurophysiological processes.
Principal Investigator
Masahisa KATSUNO (Department of Neurology, Nagoya University Graduate School of Medicine, Tokai Higher Education and Research System)
Reference
- Paper: Unpublished yet
- Patent: PCT International Patent Application pending (WO2025/063185)
Project No. BK-04061


