Advantage and Core Benefit
- First in class medicine for peritoneal dissemination, highly unmet medical needs
- Topical administration through peritoneal implantable port reduces delivery issues of ASOs
- SYT13 expression measurement in tissue or peritoneal lavage can be companion diagnostics
- Finished pre-IND PMDA (Japanese FDA) consultation
Background and Technology
Gastric cancer is the 5th most common cancer in the world and 1 million patients are currently affected. Peritoneal metastasis (PM) is the most common case when diagnosed as stage IV gastric cancer (GC) in Asian countries, as well as the most common recurrence after resection with poor effect of resection, radiation, systemic anti-cancer drug.
We identified therapeutic target Synaptotagmin13 (SYT13) among selectively expressed molecules in human samples from GC patients with PM. Since small molecules or antibodies are not a suitable approach for SYT13, we synthesized nuclease resistant amido-bridged nucleic acids (AmNA)-modified antisense oligonucleotides (ASOs), and selected ASO-4733 as a candidate.
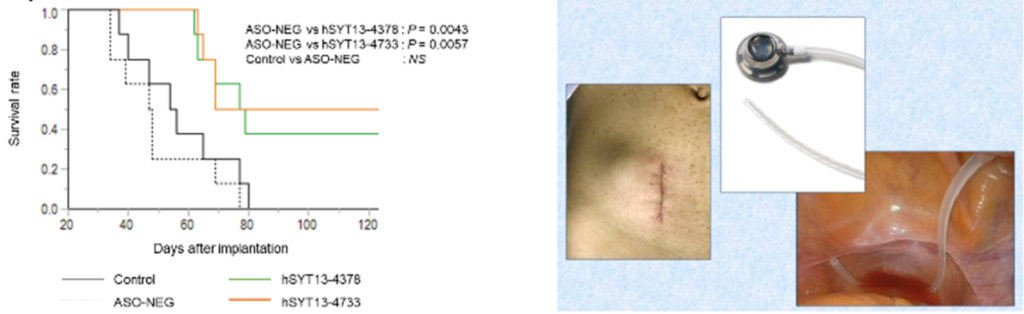
Delivery to the target tissue, as well as toxicity is the important challenge for clinical use of ASOs. Administration through peritoneal implantable port solves them (Fig. R).
Data
- SYT13 expressions in GC patient’s tissues and peritoneal lavage fluid strongly corelated with present and future PM.
- ASO-4733, was selected among 71 SYT13 ASOs.
- Intraperitoneal administration of ASO-4733 significantly reduced tumor growth, prolonged survival of GC PM model mice (Fig. L).
- Completed preclinical ADMET studies, established GMP and phase I study design.
 |
Patents and Publications
PCT/JP2020/032270: nationalized in US EP CN JP KR, JP6803572B2
https://doi.org/10.1016/j.omtn.2020.10.001
https://doi.org/10.1002/bjs.10876
https://link.springer.com/article/10.1007/s10120-024-01548-9
Researcher
Lecturer, Mitsuro Kanda, MD, PhD, Department of Gastroenterological Surgery, Nagoya University Graduate School of Medicine, Japan.
Development Plans and Expectations
First-in-human study is slated to start in 2025. Nagoya Univ. is seeking a pharmaceutical company partner, who will succeed the clinical development and commercialization.
Examples of expected indications are;
- primary treatment for unresectable advanced/recurrent gastric cancer with peritoneal dissemination,
- prophylactic administration for patients with high risk of recurrence of peritoneal dissemination after resection,
- preoperative administration for advanced gastric cancer that invades the serosa.
Project No. KJ-04010a


