Advantage and Core benefit
- Expected efficacy against many chronic inflammatory diseases, including severe asthma, eosinophilic sinusitis and inflammatory bowel disease (IBD).
 |
Background and Technology
In the process of developing allergic diseases, inflammatory immune cells infiltrate into tissues from blood vessels. If it can be possible to inhibit the infiltration of the inflammatory immune cells into the tissues, it is expected to lead to the development of new therapeutic agents for inflammatory diseases.
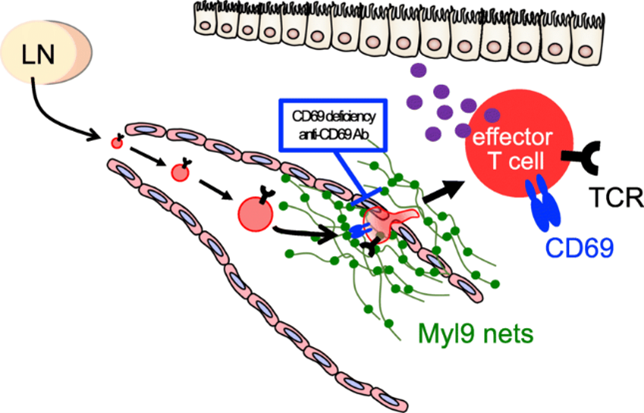
Prof. Nakayama and his colleagues at Chiba University revealed the mechanism by which deposition of Myl9 (Myl9 nets) released from activated platelets recruited inflammatory immune cells expressing CD69 to the extravascular space (the CD69-Myl9 system). Myl9 nets were observed in the lumen of the blood vessels in nasal polyps from patients with eosinophilic sinusitis or in the lung of airway inflammation model. The administration of antibodies that inhibit the interaction between CD69 and Myl9 molecules reduced the cellular infiltration in the allergic airway inflammation model, suggesting that the CD69-Myl9 system can be a therapeutic target. Myl9/12 was also highly expressed in the inflamed colon of patients with ulcerative colitis. Myl9/12 antibody treatments to a murine colitis model improved their survival and ameliorated the weight loss, suggesting the possibility of development as a therapeutic antibody against IBD.
Data
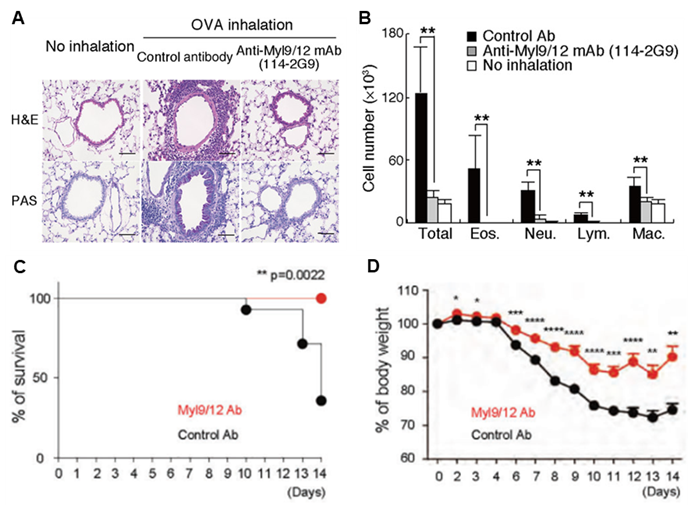
- In an allergic airway inflammation model using OVA, administration of antibodies reduced immune cell infiltration into the lungs and mucus overproduction (A), as well as the number of immune cells in the BAL (B).
- Survival (C) and weight change (D) following administration of Myl9/12 antibody to the colitis model.
 |
Patent & Publication
Basic Patent: US10100107, EP3006042, etc. Material Patent: US10513561, EP3404040, etc.
Hayashizaki et al., Sci. Immunol. (2016) DOI: 10.1126/sciimmunol.aaf9154
Yokoyama et al.,Front. Immunol. (2021) DOI: 10.3389/fimmu.2020.594297
Researcher
Dr. Motoko Kimura, Dr. Chiaki Iwamura (Chiba University)
Current Stage and Expectations
Myl9/12 human antibodies have been established. Pre-clinical and later clinical development are required to use anti-Myl9/12 antibody as a therapeutic agent. We wish to partner with pharmaceutical and biotech companies that are interested in developing drugs for chronic inflammatory diseases such as autoimmune diseases based on the Myl9/12 inhibitory antibodies.
Project No. WL-04637


