Advantages
- New solutions for areas of syncope without established prescriptions
- Novel / drug repositioning/repurposing approaches with known substances applied to antiemetic, antitussive, etc.
- By oral administration, demonstrated efficacy in rat models and in diseased canines
Background& Technology
Reflex syncope is a syncope caused by a transient decrease in cerebral blood flow and accounts for the majority of syncope attacks with no known cause. Reflex syncope can occur suddenly and is the most common cause of syncope while driving, accounting for 30 to 37% of all syncope attacks. However, because the abnormal factors in the central nervous system involved in reflex syncope have not been identified, there are only symptomatic treatments, such as refraining from going outside when fainting is likely to occur and taking blood pressure elevating drugs to prevent a drop in blood pressure caused by reflex syncope, and there is currently no causal therapy targeting the abnormal factors.
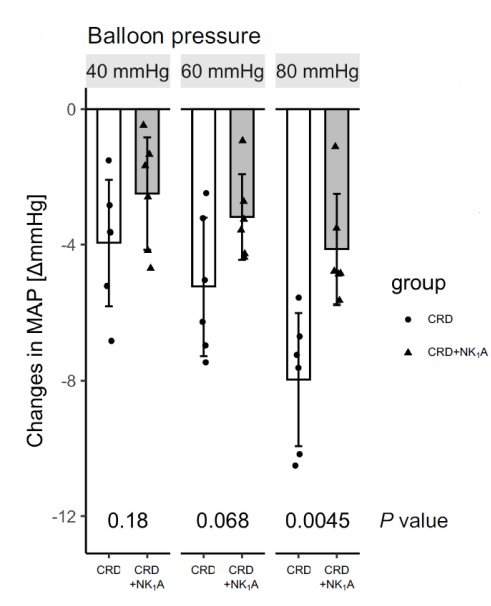
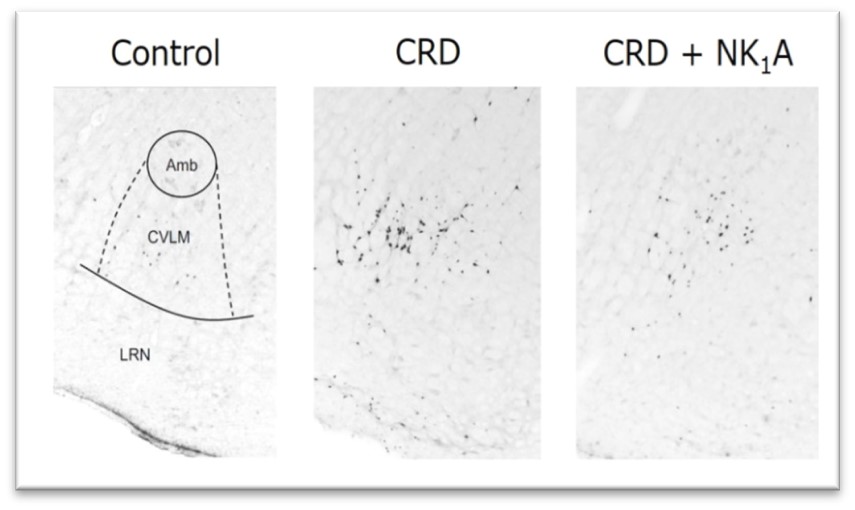
The inventors focused on the central neurokinin-1 (NK1) receptor as a factor in reflex syncope, because NK1 receptors are found in many cells in the ventral lateral area of the caudal medulla oblongata, which is involved in the inhibitory regulation of cardiovascular movement. The inventors investigated the possibility that an antagonist might alleviate reflex syncope. The inventors found that fosaprepitant, an NK1 receptor antagonist (NK1A), suppressed the hypotension induced by colorectal distention (CRD) in rats. Furthermore, the number of c-Fos immunostaining-positive cells was evaluated for neural activation, and it was confirmed that fosaprepitant suppressed the activation of the caudal medullary ventral tegmental area induced by colorectal distention. Based on these results, the NK1 antagonist maropitant was administered to dogs with reflex syncope at Azabu University Veterinary Hospital, and a decrease in the number of syncope episodes was observed.
Thus, the usefulness of NK1 receptor antagonists for the treatment of reflex syncope was demonstrated in different animals and with different antagonists.
Data
Patent
Pending (unpublished)
Applicant
Azabu University
Researcher
Professor Kensuke Orito, Department Veterinary Medicine, Azabu University
Development Phase
Current phase:
Proof of concept for suppression of reflex syncope by NK1 receptor inhibition is complete.
Next stage:
(1) Detailed mechanistic studies, formulation optimization development
(2) Clinical development
We are looking for a development partner company that can help us advance the above development. We would be happy to start with a detailed explanation and discussion of the technology.
Project No. ON-04472