Advantages and Core Benefits
- Uses relatively inexpensive catalytic materials.
- Reaction proceeds only with UV-visible light irradiation, and no heating or other energy is required.
- Reaction conditions for selective synthesis of methane, ethane, propane, ethylene, propylene, and other hydrocarbons are also under consideration.
Background and Technology
 |
 |
A carbon-neutral cycle can be realized if CO2 produced by the combustion of fossil fuels, etc., can be converted back into fuel using light energy such as sunlight (CO2 photofuel conversion). CO2 is a stable molecule and is not easy to decompose and reconstruct into fuel hydrocarbons. From a sustainability perspective, it is also important to choose a relatively inexpensive material that will allow the CO2 photofuel conversion reaction to proceed without requiring extra energy.
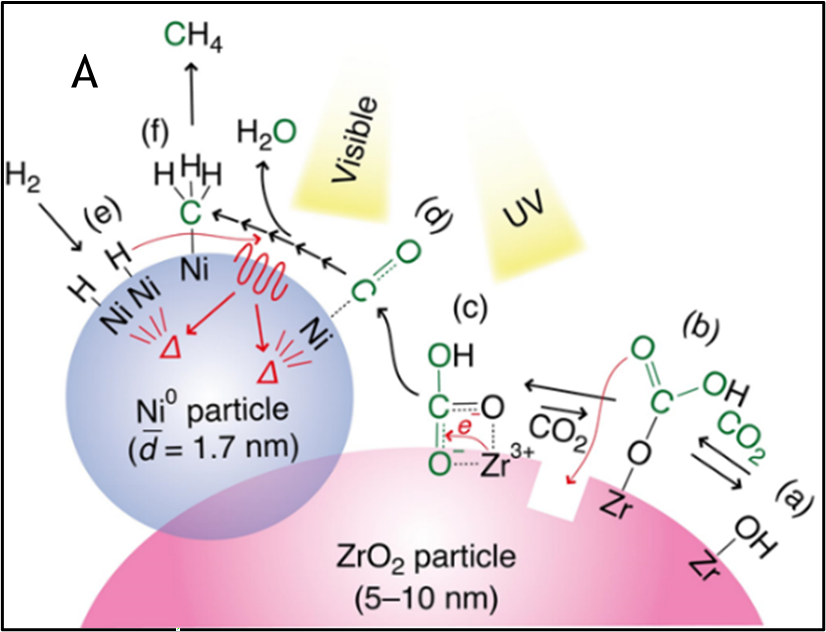
Based on the finding (https://doi.org/10.1021/jacs.8b13894) that CO2 can be reduced to carbon monoxide (CO) by a photocatalyst composed of silver nanoparticles and zirconium oxide, the inventors found that a photocatalyst composed of metallic nickel and zirconium oxide can reduce CO2 reduction to methane (CH4) by a photocatalyst composed of metallic nickel and zirconium oxide (Figure A).
- CO2 is adsorbed as HCO3 on the zirconium oxide surface.
- HCO3 is reduced by the action of zirconium oxide and ultraviolet light to produce CO.
- Hydrogen and CO react with heat on the surface of nickel to produce CH4 (https://doi.org/10.1002/anie.202016346)
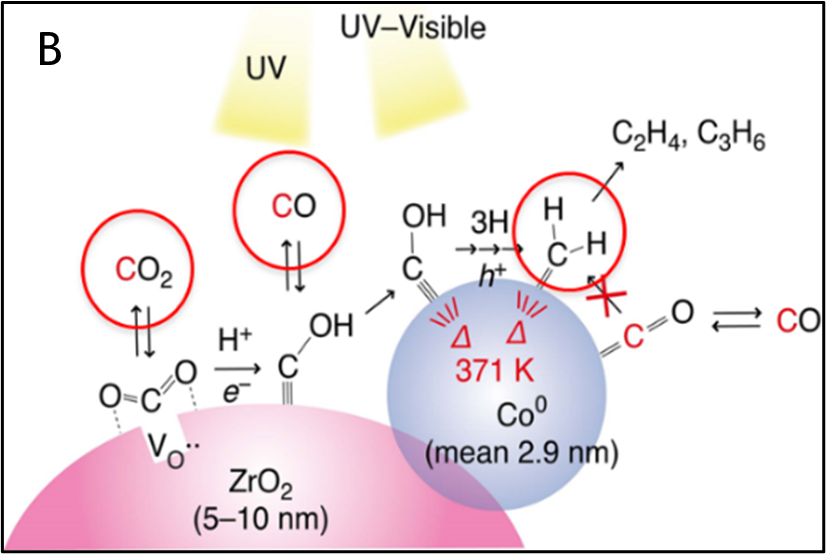
To produce more value-added C2 compounds, a zirconium oxide photocatalyst with cobalt particles supported on it was prepared to contain cobalt, which is used in typical methane conversion catalysts. After investigating a photoreaction system using this catalyst, we were successful in finding a reaction test system in which ethylene (C2H4) propylene (C3H6) was the main product (Figure B). Currently, optimization of the catalyst and reaction system is underway to improve the selective synthesis of the products, the production cost of the catalyst, and the ease of handling.
Data
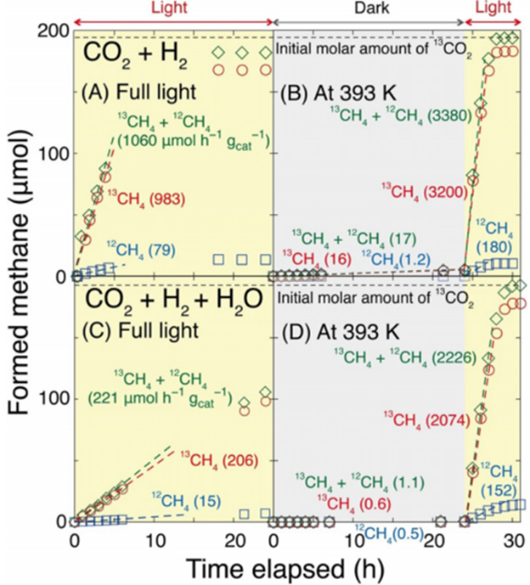
- Nickel-Zirconium Oxide Catalyst: Transition of methane (12CH4 and 13CH4) production when reacting 13CO2 (2.3 kPa) with H2 (21.7 kPa) (A) under UV/visible light or (B) under dark to UV/visible light (after 24 hours) at 393 K production. (C) and (D) are the transition when 13CO2 (2.3 kPa), H2O (2.3 kPa), and H2 (21.7 kPa) are reacted under the same conditions as in (A) and (B). The amount of catalyst and UV/visible light intensity were 0.020 g and 186 mW/cm2, respectively.
- Cobalt-Zirconium Oxide Catalyst: The reaction mechanism has been clarified and the reduction state of cobalt is important. The reaction conditions, such as the concentration of the reaction gas, have been optimized to improve ethylene production.
 |
Patents & Publication
JP2020‐172619, JP2023-009696
Researcher
Dr. Yasuo Izumi (Professor, Graduate School of Science Chiba University, Japan)
Expectations
We wish to conduct joint research with a chemical and catalyst-related company for the purpose of developing a photofuel conversion catalyst and establishing a production system. We would also like to develop a CO2 reactor incorporating this catalyst through joint research with an engineering company. We can also consider customizing the reaction products to meet the wishes of partner companies.
Project No. WL-04701


