Advantages
- Enables discovery of new vaccine seeds, nucleic acid delivery technologies, etc., libraries of candidate substances
- Detection method in which Bacterial strains with high Membrane vesicle-forming capacity are better labelled
- FACS enables the isolation of high membrane capsule-releasing bacteria in a population
Background & Technology
Membrane vesicles (MVs), produced extracellularly by bacteria, are biological microparticles 20-400 nm in diameter. Various prokaryotes, including Gram-negative bacteria, Gram-positive bacteria, and Archaea, are known to release MVs extracellularly and have been shown to contain DNA, RNA (mRNAs, rRNAs, tRNAs, sRNAs), proteins, bacterial signals (low molecular weight compounds), and antibiotics. Various functions are known, such as the delivery of immune-activating substances from the derived bacteria to host cells, activation of immune responses, and delivery of transmitter substances between microorganisms.
Since MVs produced by enteric bacteria function as carriers of immunodominant antigens and have a higher capacity to induce innate and acquired immunity than the original cells, MVs are expected to be a seed for efficient vaccine development. A group of Nobel laureates in CRISPR research has also begun to use bacterial MVs as carriers of nucleic acids (DNA/RNA) for RNA transport.
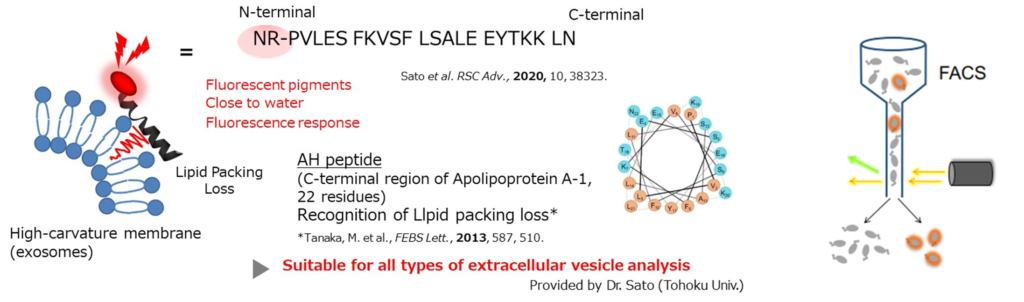
Since the establishment of mass production, etc. is necessary for the bioengineering of bacterial-derived MV, it is essential to have a technology to detect MV-producing bacteria. The conventional process of membrane vesicle formation has the problem that the MV-producing ability of bacteria cannot be detected. The inventors found that by bringing MV-producing bacteria into contact with a fluorescent probe (ApoC-NR) that selectively labels high-curvature lipid bilayers, a variety of MV-producing bacteria could be labeled with the fluorescent probe. Furthermore, we have found a method to detect high MV-producing bacteria and to fractionate MV-producing bacteria using the fluorescence of MV-producing bacteria labeled with this probe as an indicator, and have perfected the technique.
 |
Data
Labeling of membrane vesicle-producing bacteria (Buttiauxella agrestis JCM 1090T ΔtolB strain) using high curvature membrane recognition probes.
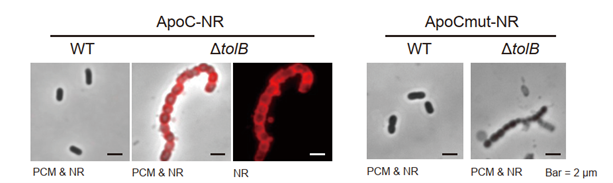 |
When ApoC-NR was used, fluorescence was not observed from bacteria in the wild-type strain with low MV production capacity, whereas fluorescence was observed from bacteria in the ΔtolB strain with excessive MV formation capacity. No fluorescence was observed from the ΔtolB strain when the control probe ApoCmut-NR was used. This indicates that detection using high curvature membrane recognition probes is feasible in MV-producing bacteria.
- ΔtolB strain is about 15 times more capable of forming MVs than the wild strain, and the released MVs are a bacterial strain with a wide variety of shapes, including small, multilamellar vesicles and multivesicular membrane vesicles.
Researcher
Shizuoka University, Graduate School of Integrated Science and Technology
Lecturer Yosuke Tashiro
Tohoku University, Graduate School of Science, Department of Chemistry
Associate Professor Yusuke Sato
Patent
Pending (unpublished)
Development Phase
Current phase:
Detection of membrane vesicle-high producing bacteria, establishment of separation process and proof of concept completed.
Next stage:
(i) Bacterial screening in target bacteria and target themes (vaccine/delivery seed screening).
(ii) Activity of the screened bacteria, performance evaluation and proof of development.
(iii) Industrialization of this process through development of mass production technology.
Both of the above can be done in collaboration/joint development with a research laboratory. (3) We hope that the partner company will take the initiative in the study of (3).
We are looking for partner companies interested in evaluation of bacteria using this technology/process or development collaboration. We would be happy to start with a detailed explanation and discussion of the technology.
Project No. ON-04647


